Autonomic Modulation for Cardiovascular Disease
- PMID: 33414727
- PMCID: PMC7783451
- DOI: 10.3389/fphys.2020.617459
Autonomic Modulation for Cardiovascular Disease
Abstract
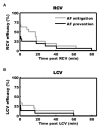
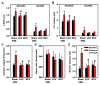
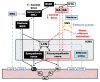
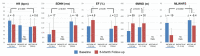
Dysfunction of the autonomic nervous system has been implicated in the pathogenesis of cardiovascular disease, including congestive heart failure and cardiac arrhythmias. Despite advances in the medical and surgical management of these entities, progression of disease persists as does the risk for sudden cardiac death. With improved knowledge of the dynamic relationships between the nervous system and heart, neuromodulatory techniques such as cardiac sympathetic denervation and vagal nerve stimulation (VNS) have emerged as possible therapeutic approaches for the management of these disorders. In this review, we present the structure and function of the cardiac nervous system and the remodeling that occurs in disease states, emphasizing the concept of increased sympathoexcitation and reduced parasympathetic tone. We review preclinical evidence for vagal nerve stimulation, and early results of clinical trials in the setting of congestive heart failure. Vagal nerve stimulation, and other neuromodulatory techniques, may improve the management of cardiovascular disorders, and warrant further study.
Keywords: arrhythmia; autonomic nervous system; heart failure; myocardial infaraction; neurocardiology; neuromodulation; sympathectomy; vagus nerve.
Copyright © 2020 Hadaya and Ardell.
Conflict of interest statement
University of California, Los Angeles has patents developed by JLA relating to cardiac neural diagnostics and therapeutics. JLA is a co-founder of NeuCures, Inc. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Autonomic modulation of ventricular electrical activity: recent developments and clinical implications.Clin Auton Res. 2021 Dec;31(6):659-676. doi: 10.1007/s10286-021-00823-4. Epub 2021 Sep 30. Clin Auton Res. 2021. PMID: 34591191 Free PMC article. Review.
-
Neuromodulation for Ventricular Tachycardia and Atrial Fibrillation: A Clinical Scenario-Based Review.JACC Clin Electrophysiol. 2019 Aug;5(8):881-896. doi: 10.1016/j.jacep.2019.06.009. Epub 2019 Aug 19. JACC Clin Electrophysiol. 2019. PMID: 31439288 Free PMC article. Review.
-
Neuromodulation for cardiac arrhythmia.Heart Rhythm. 2016 Feb;13(2):584-92. doi: 10.1016/j.hrthm.2015.10.001. Epub 2015 Oct 9. Heart Rhythm. 2016. PMID: 26440550 Review.
-
Scalable and reversible axonal neuromodulation of the sympathetic chain for cardiac control.Am J Physiol Heart Circ Physiol. 2022 Jan 1;322(1):H105-H115. doi: 10.1152/ajpheart.00568.2021. Epub 2021 Dec 3. Am J Physiol Heart Circ Physiol. 2022. PMID: 34860595 Free PMC article.
-
Vagal Stimulation and Arrhythmias.J Atr Fibrillation. 2020 Jun 30;13(1):2398. doi: 10.4022/jafib.2398. eCollection 2020 Jun-Jul. J Atr Fibrillation. 2020. PMID: 33024499 Free PMC article.
Cited by
-
Heart Rate Responses at Rest, during Exercise and after Exercise Periods in Relation to Adiposity Levels among Young Nigerian Adults.J Obes Metab Syndr. 2023 Mar 30;32(1):87-97. doi: 10.7570/jomes22055. Epub 2023 Mar 9. J Obes Metab Syndr. 2023. PMID: 36890110 Free PMC article.
-
Unpacking the multimodal, multi-scale data of the fast and slow lanes of the cardiac vagus through computational modelling.Exp Physiol. 2024 Dec;109(12):1994-2000. doi: 10.1113/EP090865. Epub 2023 Apr 30. Exp Physiol. 2024. PMID: 37120805 Free PMC article. Review.
-
Autonomic modulation of ventricular electrical activity: recent developments and clinical implications.Clin Auton Res. 2021 Dec;31(6):659-676. doi: 10.1007/s10286-021-00823-4. Epub 2021 Sep 30. Clin Auton Res. 2021. PMID: 34591191 Free PMC article. Review.
-
SGK1 Target Genes Involved in Heart and Blood Vessel Functions in PC12 Cells.Cells. 2023 Jun 15;12(12):1641. doi: 10.3390/cells12121641. Cells. 2023. PMID: 37371111 Free PMC article.
-
Heart rate variability as a digital biomarker for frailty in cardiovascular patients.J Frailty Aging. 2025 Feb;14(1):100007. doi: 10.1016/j.tjfa.2024.100007. Epub 2025 Jan 1. J Frailty Aging. 2025. PMID: 39855886 Free PMC article.
References
-
- Aaronson S. T., Carpenter L. L., Conway C. R., Reimherr F. W., Lisanby S. H., Schwartz T. L., et al. . (2013). Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 6, 631–640. 10.1016/j.brs.2012.09.013, PMID: - DOI - PubMed
-
- Ajijola O. A., Hoover D. B., Simerly T. M., Brown T. C., Yanagawa J., Biniwale R. M., et al. . (2017). Inflammation, oxidative stress, and glial cell activation characterize stellate ganglia from humans with electrical storm. JCI Insight 2:e94715. 10.1172/jci.insight.94715, PMID: - DOI - PMC - PubMed
-
- Ajijola O. A., Vaseghi M., Zhou W., Yamakawa K., Benharash P., Hadaya J., et al. . (2013). Functional differences between junctional and extrajunctional adrenergic receptor activation in mammalian ventricle. Am. J. Physiol. Heart Circ. Physiol. 304, H579–H588. 10.1152/ajpheart.00754.2012, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

