Mesenchymal Stem Cell Enhances the Function of MDSCs in Experimental Sjögren Syndrome
- PMID: 33414787
- PMCID: PMC7782428
- DOI: 10.3389/fimmu.2020.604607
Mesenchymal Stem Cell Enhances the Function of MDSCs in Experimental Sjögren Syndrome
Abstract
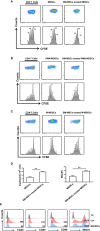
Primary Sjögren's syndrome (pSS) is a progressive systemic autoimmune disease characterized by lymphocytic infiltrates in exocrine glands, leading to the injury of salivary and lachrymal glands. Mesenchymal stem cells (MSCs) have been demonstrated to exert great potential in the treatment of various autoimmune diseases. Although MSCs have provide an effective therapeutic approach for SS treatment, the underlying mechanisms are still elusive. Our previous study has shown the reduced suppressive capacity of myeloid-derived suppressor cells (MDSCs) advanced the progression of experimental Sjögren's syndrome (ESS). In this study, we found that BM-MSCs significantly enhanced the suppressive function of MDSCs with high levels of Arginase and NO, decreased the levels of CD40, CD80, CD86, and MHC-II expression on MDSCs, thus attenuating the disease progression in ESS mice. Furthermore, the enhanced suppressive function of MDSCs was mediated by BM-MSC-secreted TGF-β, and the therapeutic effect of BM-MSCs in inhibiting ESS was almost abolished after silencing TGF-β in BM-MSCs. Taken together, our results demonstrated that BM-MSCs alleviated the ESS progression by up-regulating the immunosuppressive effect of MDSCs through TGF-β/Smad pathway, offering a novel mechanism for MSCs in the treatment of pSS.
Keywords: Sjögren’s syndrome; TGF-β; autoimmune disease; bone marrow-mesenchymal stem cell; myeloid-derived suppressor cell.
Copyright © 2020 Tian, Hong, Zhu, Zhou, Zhang, Shen, Guo, Zhang, Ai, Zhao, Rui, Xu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

