A Novel Gene Delivery Vector of Agonistic Anti-Radioprotective 105 Expressed on Cell Membranes Shows Adjuvant Effect for DNA Immunization Against Influenza
- PMID: 33414788
- PMCID: PMC7783388
- DOI: 10.3389/fimmu.2020.606518
A Novel Gene Delivery Vector of Agonistic Anti-Radioprotective 105 Expressed on Cell Membranes Shows Adjuvant Effect for DNA Immunization Against Influenza
Abstract
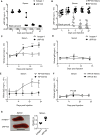
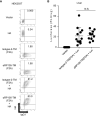
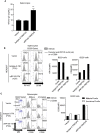
Radioprotective 105 (RP105) (also termed CD180) is an orphan and unconventional Toll-like receptor (TLR) that lacks an intracellular signaling domain. The agonistic anti-RP105 monoclonal antibody (mAb) can cross-link RP105 on B cells, resulting in the proliferation and activation of B cells. Anti-RP105 mAb also has a potent adjuvant effect, providing higher levels of antigen-specific antibodies compared to alum. However, adjuvanticity is required for the covalent link between anti-RP105 mAb and the antigen. This is a possible obstacle to immunization due to the link between anti-RP105 mAb and some antigens, especially multi-transmembrane proteins. We have previously succeeded in inducing rapid and potent recombinant mAbs in mice using antibody gene-based delivery. To simplify the covalent link between anti-RP105 mAb and antigens, we generated genetic constructs of recombinant anti-RP105 mAb (αRP105) bound to the transmembrane domain of the IgG-B cell receptor (TM) (αRP105-TM), which could enable the anti-RP105 mAb to link the antigen via the cell membrane. We confirmed the expression of αRP105-TM and the antigen hemagglutinin, which is a membrane protein of the influenza virus, on the same cell. We also found that αRP105-TM could activate splenic B cells, including both mature and immature cells, depending on the cell surface RP105 in vitro. To evaluate the adjuvanticity of αRP105-TM, we conducted DNA immunization in mice with the plasmids encoding αRP105-TM and hemagglutinin, followed by challenge with an infection of a lethal dose of an influenza virus. We then obtained partially but significantly hemagglutinin-specific antibodies and observed protective effects against a lethal dose of influenza virus infection. The current αRP105-TM might provide adjuvanticity for a vaccine via a simple preparation of the expression plasmids encoding αRP105-TM and of that encoding the target antigen.
Keywords: DNA immunization; RP105; adjuvant; agonistic antibody; antibody gene-vector delivery; cell membrane; influenza; targeting antigen to B cells.
Copyright © 2020 Yamazaki, Biswas, Kosugi, Nagashima, Inui, Tomono, Takagi, Ichimonji, Nagaoka, Ainai, Hasegawa, Chiba and Akashi-Takamura.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Synthetic Toll-Like Receptor 4 (TLR4) and TLR7 Ligands Work Additively via MyD88 To Induce Protective Antiviral Immunity in Mice.J Virol. 2017 Sep 12;91(19):e01050-17. doi: 10.1128/JVI.01050-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724768 Free PMC article.
-
The RP105/MD-1 complex is indispensable for TLR4/MD-2-dependent proliferation and IgM-secreting plasma cell differentiation of marginal zone B cells.Int Immunol. 2012 Jun;24(6):389-400. doi: 10.1093/intimm/dxs040. Epub 2012 Feb 21. Int Immunol. 2012. PMID: 22354914
-
Requirement for MD-1 in cell surface expression of RP105/CD180 and B-cell responsiveness to lipopolysaccharide.Blood. 2002 Mar 1;99(5):1699-705. doi: 10.1182/blood.v99.5.1699. Blood. 2002. PMID: 11861286
-
DNA and protein-generated chimeric molecules for delivery of influenza viral epitopes in mouse and humanized NSG transfer models.Hum Vaccin Immunother. 2024 Dec 31;20(1):2292381. doi: 10.1080/21645515.2023.2292381. Epub 2024 Jan 9. Hum Vaccin Immunother. 2024. PMID: 38193304 Free PMC article. Review.
-
State of play and clinical prospects of antibody gene transfer.J Transl Med. 2017 Jun 7;15(1):131. doi: 10.1186/s12967-017-1234-4. J Transl Med. 2017. PMID: 28592330 Free PMC article. Review.
Cited by
-
Intracellular delivery of anti-BCR/ABL antibody by PLGA nanoparticles suppresses the oncogenesis of chronic myeloid leukemia cells.J Hematol Oncol. 2021 Sep 6;14(1):139. doi: 10.1186/s13045-021-01150-x. J Hematol Oncol. 2021. PMID: 34488814 Free PMC article.
-
Human RP105 monoclonal antibody enhances antigen-specific antibody production in unique culture conditions.iScience. 2024 Aug 3;27(9):110649. doi: 10.1016/j.isci.2024.110649. eCollection 2024 Sep 20. iScience. 2024. PMID: 39246445 Free PMC article.
References
-
- Nunes-Alves C. Blood is a very unusual fluid. Nat Immunol (2016) 17(1):S5–S. 10.1038/ni.3600 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

