Mutation of SlSBPASE Aggravates Chilling-Induced Oxidative Stress by Impairing Glutathione Biosynthesis and Suppressing Ascorbate-Glutathione Recycling in Tomato Plants
- PMID: 33414794
- PMCID: PMC7783158
- DOI: 10.3389/fpls.2020.565701
Mutation of SlSBPASE Aggravates Chilling-Induced Oxidative Stress by Impairing Glutathione Biosynthesis and Suppressing Ascorbate-Glutathione Recycling in Tomato Plants
Abstract
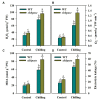
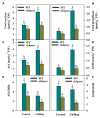
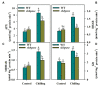
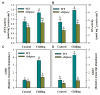
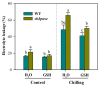
Sedoheptulose-1,7-bisphosphatase (SBPase) is a crucial enzyme for photosynthetic carbon assimilation in the Calvin-Benson cycle. Previous studies have shown that overexpression of SBPase is advantageous to chilling tolerance in plants; however, the mechanisms of SBPase acting in the improvement of chilling tolerance remain largely unknown. In the present study, we aimed to uncover the essential role of SBPase in the response of tomato plants to oxidative stress induced by low temperature. To fulfill that, we performed an array of comparative studies between slsbpase mutant plants that we previously generated using CRISPR/Cas9 genome editing system and their wild-type counterparts under chilling stress. It was observed that following a 24 h chilling treatment, slsbpase mutant plants accumulated higher levels of reactive oxygen species (ROS) than wild-type plants and consequently, more severe lipid peroxidation occurred in slsbpase plants. Activity assay of antioxidant enzymes showed that mutation in SlSBPASE significantly decreased activities of peroxidase (POD) and ascorbate peroxidase (APX), but surprisingly did not significantly alter activities of superoxide dismutase (SOD) and catalase (CAT) under the chilling condition. Notably, mutation in SlSBPASE reduced the contents of total ascorbate (AsA) and total glutathione (GSH) and suppressed the recycling of AsA and GSH in chilling-stressed tomato plants. In addition, activities of two GSH biosynthetic enzymes (gamma-glutamylcysteine synthetase and glutathione synthetase) and transcript abundance of their coding genes (GSH1 and GSH2) were markedly reduced in slsbpase mutant plants in comparison with those in wild-type plants under chilling stress. Furthermore, exogenous GSH remarkably mitigated chilling damage in slsbpase plants. Collectively, these results support that mutation in SlSBPASE aggravates chilling-induced oxidative stress by suppressing GSH biosynthesis and AsA-GSH recycling and suggest that SBPase is required for optimal response to chilling stress in tomato plants. The findings also shed light on the idea to mitigate chilling-induced damages by genetically manipulating a photosynthetic enzyme in plants.
Keywords: SBPase; ascorbate; chilling stress; glutathione; oxidative stress; reactive oxygen species; tomato.
Copyright © 2020 Wang, Ding and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Knockout of SlSBPASE Suppresses Carbon Assimilation and Alters Nitrogen Metabolism in Tomato Plants.Int J Mol Sci. 2018 Dec 14;19(12):4046. doi: 10.3390/ijms19124046. Int J Mol Sci. 2018. PMID: 30558146 Free PMC article.
-
Melatonin Mitigates Chilling-Induced Oxidative Stress and Photosynthesis Inhibition in Tomato Plants.Antioxidants (Basel). 2020 Mar 6;9(3):218. doi: 10.3390/antiox9030218. Antioxidants (Basel). 2020. PMID: 32155702 Free PMC article.
-
Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants.Sci Rep. 2016 Sep 2;6:32741. doi: 10.1038/srep32741. Sci Rep. 2016. PMID: 27586456 Free PMC article.
-
Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress.Antioxidants (Basel). 2019 Sep 9;8(9):384. doi: 10.3390/antiox8090384. Antioxidants (Basel). 2019. PMID: 31505852 Free PMC article. Review.
-
Ascorbic Acid-The Little-Known Antioxidant in Woody Plants.Antioxidants (Basel). 2019 Dec 14;8(12):645. doi: 10.3390/antiox8120645. Antioxidants (Basel). 2019. PMID: 31847411 Free PMC article. Review.
Cited by
-
Jasmonate Positively Regulates Cold Tolerance by Promoting ABA Biosynthesis in Tomato.Plants (Basel). 2022 Dec 22;12(1):60. doi: 10.3390/plants12010060. Plants (Basel). 2022. PMID: 36616188 Free PMC article.
-
Exogenous 6-Benzyladenine Improves Waterlogging Tolerance in Maize Seedlings by Mitigating Oxidative Stress and Upregulating the Ascorbate-Glutathione Cycle.Front Plant Sci. 2021 Sep 3;12:680376. doi: 10.3389/fpls.2021.680376. eCollection 2021. Front Plant Sci. 2021. PMID: 34539688 Free PMC article.
-
Jasmonate: A Hormone of Primary Importance for Temperature Stress Response in Plants.Plants (Basel). 2023 Dec 6;12(24):4080. doi: 10.3390/plants12244080. Plants (Basel). 2023. PMID: 38140409 Free PMC article. Review.
-
Dynamic characteristics and functional analysis provide new insights into the role of GauERF105 for resistance against Verticillium dahliae in cotton.BMC Plant Biol. 2023 Oct 18;23(1):501. doi: 10.1186/s12870-023-04455-w. BMC Plant Biol. 2023. PMID: 37848871 Free PMC article.
-
Ascorbic Acid-Induced Photosynthetic Adaptability of Processing Tomatoes to Salt Stress Probed by Fast OJIP Fluorescence Rise.Front Plant Sci. 2021 Aug 16;12:594400. doi: 10.3389/fpls.2021.594400. eCollection 2021. Front Plant Sci. 2021. PMID: 34484251 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

