Patient perspectives on living with severe asthma in Denmark and Sweden
- PMID: 33414901
- PMCID: PMC7751392
- DOI: 10.1080/20018525.2020.1856024
Patient perspectives on living with severe asthma in Denmark and Sweden
Abstract
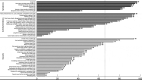
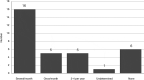
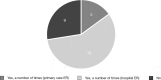
Background: Severe asthma has an acknowledged impact on health-related quality of life (HRQOL) and is associated with substantial health care costs. This study aimed to investigate the patients' own experiences of the disease, perceptions of HRQOL, and awareness of disease management. Methods: This study included severe asthma patients in Sweden and Denmark. A quantitative Web-based survey and qualitative in-depth interviews (IDIs) were conducted. The survey included St. George's Respiratory Questionnaire (SGRQ), Asthma Control Test (ACT), Work Productivity and Activity Impairment (WPAI), and a study-specific questionnaire on quality of care and disease awareness. Telephone-based IDIs were conducted by medical interviewers following a semi-structured interview guide. Results: A total of 93 patients participated in the Web survey, and 33 participated in the IDIs. In the survey, the vast majority (77%; 72/93) had uncontrolled asthma (ACT<20). Mean total SGRQ score was 47.4 (59.7 symptom, 53.7 activity, 39.9 impact scores). Nearly 60% were treated in primary care. The IDIs revealed a long path to diagnosis, substantial and constant need for adaptations because of disease limitations, high burden on family members, social restrictions, and sick leaves and income losses. Patient awareness about guidelines, treatment goals, and available therapies was poor, and a low level of satisfaction by primary health care was seen. Conclusions: The vast majority of this severe asthma population had uncontrolled asthma and poor access to lung expert physicians. Impaired HRQOL despite patients' adaptations was indicated. These findings highlight the need for structured patient education and greater access to units with disease-specific knowledge.
Keywords: Asthma control; patient perspective; patient-reported outcomes; severe asthma.
© 2020 Informa UK Limited, trading as Taylor & Francis.
Conflict of interest statement
GP has received Principal Investigator fees by AstraZeneca. She has also participated in advisory boards for AstraZeneca and Novartis. AT has participated in advisory boards and provided medical expertise against remuneration for medical staff in the fields of allergy, asthma and COPD, sponsored by AstraZeneca, GSK, Boehringer Ingelheim, MSD, ALK, Novartis, Mundipharma, Nycomed, Sanofi, Teva, Almirall Nordic, and AGA. JB has participated in studies sponsored by AstraZeneca and Viscogel. He has been Scientific consultant for AstraZeneca. GT are employed by AstraZeneca. KR has collaborated with AstraZeneca, GSK, Chiesi, Boehringer-ingelheim, Mundipharma and Teva, supporting education and projects in respiratory medicine. He has also participated in advisory boards for the same companies.
Figures
References
-
- Global Asthma Network. The global asthma report 2019. Available from: GINA-2019-main-report-June-2019-wms.pdf
-
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–7. - PubMed
-
- O’Byrne PM, Pedersen S, Schatz M, et al. The poorly explored impact of uncontrolled asthma. Chest. 2013;143(2):511–523. - PubMed
-
- von Bulow A, Kriegbaum M, Backer V, et al. The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract. 2014;2(6):759–767. - PubMed
LinkOut - more resources
Full Text Sources