Decellularized pulp matrix as scaffold for mesenchymal stem cell mediated bone regeneration
- PMID: 33414903
- PMCID: PMC7750895
- DOI: 10.1177/2041731420981672
Decellularized pulp matrix as scaffold for mesenchymal stem cell mediated bone regeneration
Abstract
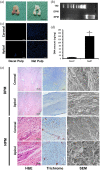
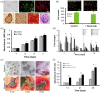
Scaffolds that are used for bone repair should provide an adequate environment for biomineralization by mesenchymal stem cells (MSCs). Recently, decellularized pulp matrices (DPM) have been utilized in endodontics for their high regenerative potential. Inspired by the dystrophic calcification on the pulp matrix known as pulp stone, we developed acellular pulp bioscaffolds and examined their potential in facilitating MSCs mineralization for bone defect repair. Pulp was decellularized, then retention of its structural integrity was confirmed by histological, mechanical, and biochemical evaluations. MSCs were seeded and proliferation, osteogenic gene expression, and biomineralization were assessed to verify DPM's osteogenic effects in vitro. MicroCT, energy-dispersive X-ray (EDX), and histological analyses were used to confirm that DPM seeded with MSCs result in greater mineralization on rat critical-sized defects than that without MSCs. Overall, our study proves DPM's potential to serve as a scaffolding material for MSC-mediated bone regeneration for future craniofacial bone tissue engineering.
Keywords: Decellularized pulp matrix; biomineralization; critical sized defect; dystrophic calcification; mesenchymal stem cells.
© The Author(s) 2020.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




Similar articles
-
The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects.Stem Cell Res Ther. 2017 May 25;8(1):122. doi: 10.1186/s13287-017-0574-6. Stem Cell Res Ther. 2017. PMID: 28545565 Free PMC article.
-
Decellularized cartilage-derived matrix as substrate for endochondral bone regeneration.Tissue Eng Part A. 2015 Feb;21(3-4):694-703. doi: 10.1089/ten.TEA.2014.0117. Epub 2014 Nov 20. Tissue Eng Part A. 2015. PMID: 25316202
-
Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells.Sci Rep. 2016 Dec 9;6:38814. doi: 10.1038/srep38814. Sci Rep. 2016. PMID: 27934940 Free PMC article.
-
Pulp-dentin Regeneration: Current State and Future Prospects.J Dent Res. 2015 Nov;94(11):1544-51. doi: 10.1177/0022034515601658. Epub 2015 Aug 26. J Dent Res. 2015. PMID: 26310721 Review.
-
Dental Pulp Tissue Engineering Using Mesenchymal Stem Cells: a Review with a Protocol.Stem Cell Rev Rep. 2018 Oct;14(5):668-676. doi: 10.1007/s12015-018-9826-9. Stem Cell Rev Rep. 2018. PMID: 29804171 Review.
Cited by
-
Fibroblasts inhibit osteogenesis by regulating nuclear-cytoplasmic shuttling of YAP in mesenchymal stem cells and secreting DKK1.Biol Res. 2024 Jan 20;57(1):4. doi: 10.1186/s40659-023-00481-y. Biol Res. 2024. PMID: 38245803 Free PMC article.
-
The Regenerative Potential of Decellularized Dental Pulp Extracellular Matrix: A Systematic Review.Materials (Basel). 2022 Sep 14;15(18):6386. doi: 10.3390/ma15186386. Materials (Basel). 2022. PMID: 36143698 Free PMC article. Review.
-
Preparation and characterization of bovine dental pulp-derived extracellular matrix hydrogel for regenerative endodontic applications: an in vitro study.BMC Oral Health. 2024 Oct 24;24(1):1281. doi: 10.1186/s12903-024-05004-z. BMC Oral Health. 2024. PMID: 39448989 Free PMC article.
-
Synergistically Promoting Bone Regeneration by Icariin-Incorporated Porous Microcarriers and Decellularized Extracellular Matrix Derived From Bone Marrow Mesenchymal Stem Cells.Front Bioeng Biotechnol. 2022 Apr 7;10:824025. doi: 10.3389/fbioe.2022.824025. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35464719 Free PMC article.
-
Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration.Biomolecules. 2023 Apr 14;13(4):673. doi: 10.3390/biom13040673. Biomolecules. 2023. PMID: 37189420 Free PMC article. Review.
References
-
- Johari B, Kadivar M, Lak S, et al. Osteoblast-seeded bioglass/gelatin nanocomposite: a promising bone substitute in critical-size calvarial defect repair in rat. Int J Artif Organs 2016; 39(10): 524–533. - PubMed
-
- Oryan A, Kamali A, Moshiri A, et al. Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells Tissues Organs 2017; 204(2): 59–83. - PubMed
LinkOut - more resources
Full Text Sources

