CD4+ T cell exhaustion leads to adoptive transfer therapy failure which can be prevented by immune checkpoint blockade
- PMID: 33414997
- PMCID: PMC7783768
CD4+ T cell exhaustion leads to adoptive transfer therapy failure which can be prevented by immune checkpoint blockade
Abstract
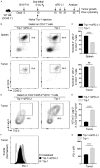
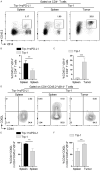
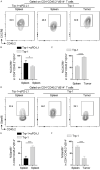
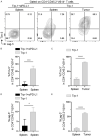
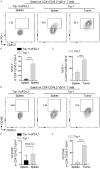
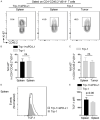
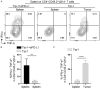
Cytotoxic CD8+ T cell exhaustion is one of the mechanisms underlying the tumor immune escape. The paradigm-shifting immune checkpoint therapy can mitigate CD8+ T lymphocyte exhaustion, reinvigorate the anticancer immunity, and achieve durable tumor regression for some patients. Emerging evidence indicates that CD4+ T lymphocytes also have a critical role in anticancer immunity, either by directly applying cytotoxicity toward cancer cells or as a helper to augment CD8+ T cell cytotoxicity. Whether anticancer CD4+ T lymphocytes undergo exhaustion during immunotherapy of solid tumors remains unknown. Here we report that melanoma antigen TRP-1/gp75-specific CD4+ T lymphocytes exhibit an exhaustion phenotype after being adoptively transferred into mice bearing large subcutaneous melanoma. Exhaustion of these CD4+ T lymphocytes is accompanied with reduced cytokine release and increased expression of inhibitory receptors, resulting in loss of tumor control. Importantly, we demonstrate that PD-L1 immune checkpoint blockade can prevent exhaustion, induce proliferation of the CD4+ T lymphocytes, and consequently prevent tumor recurrence. Therefore, when encountering an excessive amount of tumor antigens, tumor-reactive CD4+ T lymphocytes also enter the exhaustion state, which can be prevented by immune checkpoint blockade. Our results highlight the importance of tumor-specific CD4+ T lymphocytes in antitumor immunity and suggest that the current immune checkpoint blockade therapy may achieve durable anticancer efficacy by rejuvenating both tumor antigen-specific CD8+ T lymphocytes and CD4+ T lymphocytes.
Keywords: CD4+ T cells; adoptive transfer therapy; exhaustion; immune checkpoint blockade.
AJCR Copyright © 2020.
Conflict of interest statement
None.
Figures

Similar articles
-
PD-1 blockade restores helper activity of tumor-infiltrating, exhausted PD-1hiCD39+ CD4 T cells.JCI Insight. 2021 Jan 25;6(2):e142513. doi: 10.1172/jci.insight.142513. JCI Insight. 2021. PMID: 33332284 Free PMC article.
-
PD-1 blockade therapy augments the antitumor effects of lymphodepletion and adoptive T cell transfer.Cancer Immunol Immunother. 2022 Jun;71(6):1357-1369. doi: 10.1007/s00262-021-03078-0. Epub 2021 Oct 17. Cancer Immunol Immunother. 2022. PMID: 34657194 Free PMC article.
-
PD-L1-Independent Mechanisms Control the Resistance of Melanoma to CD4+ T Cell Adoptive Immunotherapy.J Immunol. 2018 May 1;200(9):3304-3311. doi: 10.4049/jimmunol.1701617. Epub 2018 Mar 30. J Immunol. 2018. PMID: 29602773
-
CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review.J Cell Physiol. 2019 Jun;234(6):8509-8521. doi: 10.1002/jcp.27782. Epub 2018 Nov 22. J Cell Physiol. 2019. PMID: 30520029 Review.
-
Coinhibitory Receptor Expression and Immune Checkpoint Blockade: Maintaining a Balance in CD8+ T Cell Responses to Chronic Viral Infections and Cancer.Front Immunol. 2017 Sep 29;8:1215. doi: 10.3389/fimmu.2017.01215. eCollection 2017. Front Immunol. 2017. PMID: 29033936 Free PMC article. Review.
Cited by
-
Clinical implications of T cell exhaustion for cancer immunotherapy.Nat Rev Clin Oncol. 2022 Dec;19(12):775-790. doi: 10.1038/s41571-022-00689-z. Epub 2022 Oct 10. Nat Rev Clin Oncol. 2022. PMID: 36216928 Free PMC article. Review.
-
Bladder cancer intrinsic LRFN2 drives anticancer immunotherapy resistance by attenuating CD8+ T cell infiltration and functional transition.J Immunother Cancer. 2023 Oct;11(10):e007230. doi: 10.1136/jitc-2023-007230. J Immunother Cancer. 2023. PMID: 37802603 Free PMC article.
-
Multiomic profiling of chronically activated CD4+ T cells identifies drivers of exhaustion and metabolic reprogramming.PLoS Biol. 2024 Dec 17;22(12):e3002943. doi: 10.1371/journal.pbio.3002943. eCollection 2024 Dec. PLoS Biol. 2024. PMID: 39689157 Free PMC article.
-
The role of CD4+ T cells in tumor and chronic viral immune responses.MedComm (2020). 2023 Oct 10;4(5):e390. doi: 10.1002/mco2.390. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37829505 Free PMC article. Review.
-
LncRNA SNHG4 promotes malignant biological behaviors and immune escape of colorectal cancer cells by regulating the miR-144-3p/MET axis.Am J Transl Res. 2021 Oct 15;13(10):11144-11161. eCollection 2021. Am J Transl Res. 2021. PMID: 34786048 Free PMC article.
References
-
- Sekine T, Perez-Potti A, Nguyen S, Gorin JB, Wu VH, Gostick E, Llewellyn-Lacey S, Hammer Q, Falck-Jones S, Vangeti S, Yu M, Smed-Sörensen A, Gaballa A, Uhlin M, Sandberg JK, Brander C, Nowak P, Goepfert PA, Price DA, Betts MR, Buggert M. TOX is expressed by exhausted and polyfunctional human effector memory CD8(+) T cells. Sci Immunol. 2020;5:eaba7918. - PubMed
-
- Hudson WH, Gensheimer J, Hashimoto M, Wieland A, Valanparambil RM, Li P, Lin JX, Konieczny BT, Im SJ, Freeman GJ, Leonard WJ, Kissick HT, Ahmed R. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1(+) stem-like CD8(+) T cells during chronic infection. Immunity. 2019;51:1043–1058. e1044. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
