A new compound targets the AF-1 of androgen receptor and decreases its activity and protein levels in prostate cancer cells
- PMID: 33415022
- PMCID: PMC7783748
A new compound targets the AF-1 of androgen receptor and decreases its activity and protein levels in prostate cancer cells
Abstract
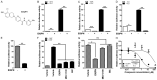
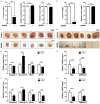
Increased expression levels of constitutively active androgen receptor splice variants (AR-Vs) cause alterations in AR signaling, resulting in drug resistance and failed hormone therapy among patients with advanced prostate cancers. Several available compounds targeting the androgen axis and AR signaling have not demonstrated efficacy in preventing prostate cancer recurrence. Here, we investigated whether a new agent, 6-[6-ethoxy-5-ispropoxy-3,4-dihydroisoquinolin-2[1H)-yl]-N-[6-methylpyridin-2-yl]nicotinamide (EIQPN), has the potential for treating advanced prostate cancer. EIQPN interacted with the AR-activation fragment-1 (AF-1) domain and blocked its androgen-independent activity, robustly decreased the protein levels of AR and variants in prostate cancer cells by inducing AR protein degradation, and inhibited the androgen-independent proliferation of various AR-positive prostate cancer cells. In xenograft mouse models, EIQPN blocked the tumor growth of androgen-independent prostate cancer cells. Overall, these findings indicate that EIQPN could serve as a novel therapeutic agent for advanced recurrent prostate cancers.
Keywords: Prostate cancer; androgen receptor; androgen-independent activity; protein degradation; splice variant.
AJCR Copyright © 2020.
Conflict of interest statement
None.
Figures




References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- Mohler JL. Castration-recurrent prostate cancer is not androgen-independent. Adv Exp Med Biol. 2008;617:223–234. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
