Complete Pathologic Responses With Immunotherapy in Metastatic Renal Cell Carcinoma: Case Reports
- PMID: 33415079
- PMCID: PMC7783360
- DOI: 10.3389/fonc.2020.609235
Complete Pathologic Responses With Immunotherapy in Metastatic Renal Cell Carcinoma: Case Reports
Abstract
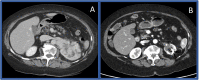
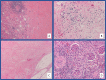
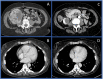
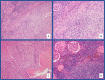
Immunotherapy-based combinations have become standard of care in advanced renal cell carcinoma (RCC). Despite the potential for complete radiographic response, complete pathologic responses have been rarely reported. We present two cases of confirmed complete pathologic response to immunotherapy despite residual radiographic abnormalities. The first case describes a 68-year-old female with metastatic RCC who was treated with upfront pembrolizumab plus axitinib. She underwent nephrectomy after 15 doses of pembrolizumab with pathology revealing no evidence of viable tumor. To our knowledge, this is the first reported case of a complete pathologic response with pembrolizumab in metastatic RCC. The second case describes a 64-year-old female with metastatic RCC who was treated with second-line nivolumab after progression on cabozantinib. After 13 doses of nivolumab, she underwent nephrectomy with pathology revealing no evidence of viable tumor. These cases highlight the potential for scar tissue, fibrosis, and necrosis to persist radiographically after treatment with immunotherapy despite the absence of viable tumor cells.
Keywords: case reports; immunotherapy; kidney neoplasms; pathologic complete responders; pembrolizumab.
Copyright © 2020 Tucker, Beckermann, Gordetsky, Giannico, Davis and Rini.
Conflict of interest statement
KB, research funding to institution: Bristol-Myers Squibb for a young investigator grant; serves on advisory board for MedOnc Live and Exelexis. ND, research funding to institution: Astra-Zeneca, Hoffman-LaRoche, Pfizer, Merck, Incyte, Immunomedics, Mirati Therapeutics, Seattle Genetics, Exelixis, Taris Biomedical, Bristol-Myers Squibb, Jounce Therapeutics; Travel: Calithera Biosciences. BR, research funding to institution: Pfizer, Merck, GNE/Roche, Aveo, Astra-Zeneca, Bristol-Myers Squibb, Exelixis; consulting: BMS, Pfizer, GNE/Roche, Aveo, Synthorx, Compugen, Merck, Corvus, Surface Oncology, 3DMedicines, Arravive, Alkermes, Arrowhead, GSK; stock: PTC therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial [published online ahead of print, 2020 Jul 16]. Cancer (2020) 126 (18):4156–67. 10.1002/cncr.33033. 10.1002/cncr.33033. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

