Advances in Nutritional Epigenetics-A Fresh Perspective for an Old Idea. Lessons Learned, Limitations, and Future Directions
- PMID: 33415317
- PMCID: PMC7750768
- DOI: 10.1177/2516865720981924
Advances in Nutritional Epigenetics-A Fresh Perspective for an Old Idea. Lessons Learned, Limitations, and Future Directions
Abstract
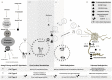
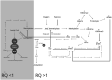
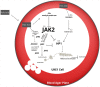
Nutritional epigenetics is a rapidly expanding field of research, and the natural modulation of the genome is a non-invasive, sustainable, and personalized alternative to gene-editing for chronic disease management. Genetic differences and epigenetic inflexibility resulting in abnormal gene expression, differential or aberrant methylation patterns account for the vast majority of diseases. The expanding understanding of biological evolution and the environmental influence on epigenetics and natural selection requires relearning of once thought to be well-understood concepts. This research explores the potential for natural modulation by the less understood epigenetic modifications such as ubiquitination, nitrosylation, glycosylation, phosphorylation, and serotonylation concluding that the under-appreciated acetylation and mitochondrial dependant downstream epigenetic post-translational modifications may be the pinnacle of the epigenomic hierarchy, essential for optimal health, including sustainable cellular energy production. With an emphasis on lessons learned, this conceptional exploration provides a fresh perspective on methylation, demonstrating how increases in environmental methane drive an evolutionary down regulation of endogenous methyl groups synthesis and demonstrates how epigenetic mechanisms are cell-specific, making supplementation with methyl cofactors throughout differentiation unpredictable. Interference with the epigenomic hierarchy may result in epigenetic inflexibility, symptom relief and disease concomitantly and may be responsible for the increased incidence of neurological disease such as autism spectrum disorder.
Keywords: 5-methyltetrahydrofolate; DNA; MTHFR; acetylation; autism spectrum disorder; carbon; environment; epigenetics; evolution; folic acid; future directions; gene expression; glycosylation; histone; limitations; methane; methylation; methylenetetrahydrofolate reductase; natural selection; neural tube defects; nitrosylation; nutrition; nutritional epigenetics; one carbon etabolism; phosphorylation; pollution; single nucleotide variant; ubiquitination.
© The Author(s) 2020.
Conflict of interest statement
Declaration of conflicting interests:The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Epigenomics in stress tolerance of plants under the climate change.Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review.
-
Acute high folic acid treatment in SH-SY5Y cells with and without MTHFR function leads to gene expression changes in epigenetic modifying enzymes, changes in epigenetic marks, and changes in dendritic spine densities.PLoS One. 2021 Jan 7;16(1):e0245005. doi: 10.1371/journal.pone.0245005. eCollection 2021. PLoS One. 2021. PMID: 33411826 Free PMC article.
-
[Epigenetics of schizophrenia: a review].Encephale. 2014 Oct;40(5):380-6. doi: 10.1016/j.encep.2014.06.005. Epub 2014 Aug 12. Encephale. 2014. PMID: 25127897 Review. French.
-
Asthma epigenetics.Adv Exp Med Biol. 2014;795:183-99. doi: 10.1007/978-1-4614-8603-9_11. Adv Exp Med Biol. 2014. PMID: 24162909 Review.
-
An Update of Epigenetic Drugs for the Treatment of Cancers and Brain Diseases: A Comprehensive Review.Genes (Basel). 2023 Apr 6;14(4):873. doi: 10.3390/genes14040873. Genes (Basel). 2023. PMID: 37107631 Free PMC article. Review.
Cited by
-
Exploring fatty acids from royal jelly as a source of histone deacetylase inhibitors: from the hive to applications in human well-being and health.Epigenetics. 2024 Dec;19(1):2400423. doi: 10.1080/15592294.2024.2400423. Epub 2024 Sep 10. Epigenetics. 2024. PMID: 39255363 Free PMC article.
-
Vitamin D as a Nutri-Epigenetic Factor in Autoimmunity-A Review of Current Research and Reports on Vitamin D Deficiency in Autoimmune Diseases.Nutrients. 2022 Oct 14;14(20):4286. doi: 10.3390/nu14204286. Nutrients. 2022. PMID: 36296970 Free PMC article. Review.
-
Epigenetic Connections of the TRPA1 Ion Channel in Pain Transmission and Neurogenic Inflammation - a Therapeutic Perspective in Migraine?Mol Neurobiol. 2023 Oct;60(10):5578-5591. doi: 10.1007/s12035-023-03428-2. Epub 2023 Jun 16. Mol Neurobiol. 2023. PMID: 37326902 Free PMC article. Review.
-
The epigenetic potential of vitamin K2 in brain health.Epigenomics. 2025 Jul;17(10):681-690. doi: 10.1080/17501911.2025.2518916. Epub 2025 Jun 12. Epigenomics. 2025. PMID: 40506698 Free PMC article. Review.
-
Genetics and Epigenetics of One-Carbon Metabolism Pathway in Autism Spectrum Disorder: A Sex-Specific Brain Epigenome?Genes (Basel). 2021 May 20;12(5):782. doi: 10.3390/genes12050782. Genes (Basel). 2021. PMID: 34065323 Free PMC article. Review.
References
-
- Kirke PN, Molloy AM, Daly LE, Burke H, Weir DC, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;86:703-708. - PubMed
-
- Cavaliere G. WHO Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing - Background Paper the Ethics of Human Genome Editing. WHO; 2019:1-37.
LinkOut - more resources
Full Text Sources

