Safety and efficacy of tolcapone in Parkinson's disease: systematic review
- PMID: 33415500
- PMCID: PMC8128808
- DOI: 10.1007/s00228-020-03081-x
Safety and efficacy of tolcapone in Parkinson's disease: systematic review
Abstract
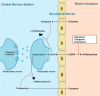
Purpose: Tolcapone is an efficacious catechol-O-methyltransferase inhibitor for Parkinson's disease (PD). However, safety issues hampered its use in clinical practice. We aimed to provide evidence of safety and efficacy of tolcapone by a systematic literature review to support clinicians' choices in the use of an enlarging PD therapeutic armamentarium.
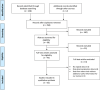
Methods: We searched PubMed for studies on PD patients treated with tolcapone, documenting the following outcomes: liver enzyme, adverse events (AEs), daily Off-time, levodopa daily dose, unified Parkinson's disease rating scale (UPDRS) part-III, quality of life (QoL), and non-motor symptoms. FAERS and EudraVigilance databases for suspected AEs were interrogated for potential additional cases of hepatotoxicity.
Results: Thirty-two studies were included, for a total of 4780 patients treated with tolcapone. Pertaining safety, 0.9% of patients showed liver enzyme elevation > 2. Over 23 years, we found 7 cases of severe liver injury related to tolcapone, 3 of which were fatal. All fatal cases did not follow the guidelines for liver function monitoring. FAERS and EudraVigilance database search yielded 61 reports of suspected liver AEs possibly related to tolcapone. Pertaining efficacy, the median reduction of hours/day spent in Off was 2.1 (range 1-3.2), of levodopa was 108.9 mg (1-251.5), of "On" UPDRS-III was 3.6 points (1.1-6.5). Most studies reported a significant improvement of QoL and non-motor symptoms.
Conclusion: Literature data showed the absence of relevant safety concerns of tolcapone when strict adherence to hepatic function monitoring is respected. Given its high efficacy on motor fluctuations, tolcapone is probably an underutilized tool in the therapeutic PD armamentarium.
Keywords: Catechol-O-methyltransferase; Efficacy; Liver; Parkinson’s disease; Safety; Tolcapone.
Conflict of interest statement
Dr. Artusi received travel grants from Zambon and Abbvie, and educational grants from Ralpharma and Neuraxpharm.
Dr. Sarro reports no financial disclosures.
Dr. Imbalzano reports no financial disclosures.
Dr. Fabbri received speaker honoraria from Abbvie.
Prof. Lopiano received honoraria for lecturing and travel grants from, UCB Pharma, AbbVie, DOC, Zambon and Bial.
Figures
References
-
- Kurth MC, Adler CH (1998) COMT inhibition: a new treatment strategy for Parkinson's disease. Neurology 50:S3–S14 - PubMed
-
- Stowe R, Ives N, Clarke CE, et al. Evaluation of the efficacy and safety of adjuvant treatment to levodopa therapy in Parkinson s disease patients with motor complications. Cochrane Database Syst Rev. 2010;7:CD007166. - PubMed
-
- Fabbri M, Ferreira JJ, Lees A, Stocchi F, Poewe W, Tolosa E, Rascol O. Opicapone for the treatment of Parkinson’s disease: a review of a new licensed medicine. Mov Disord. 2018;33:1528–1539. - PubMed
-
- Ceravolo R, Piccini P, Bailey DL, Jorga KM, Bryson H, Brooks DJ. 18F-dopa PET evidence that tolcapone acts as a central COMT inhibitor in Parkinson’s disease. Synapse. 2002;43:201–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical