Proliferative Verrucous Leukoplakia: An Expert Consensus Guideline for Standardized Assessment and Reporting
- PMID: 33415517
- PMCID: PMC8134585
- DOI: 10.1007/s12105-020-01262-9
Proliferative Verrucous Leukoplakia: An Expert Consensus Guideline for Standardized Assessment and Reporting
Abstract
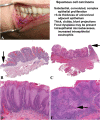
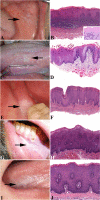
The many diverse terms used to describe the wide spectrum of changes seen in proliferative verrucous leukoplakia (PVL) have resulted in disparate clinical management. The objective of this study was to produce an expert consensus guideline for standardized assessment and reporting by pathologists diagnosing PVL related lesions. 299 biopsies from 84 PVL patients from six institutions were selected from patients who had multifocal oral leukoplakic lesions identified over several years (a minimum follow-up period of 36 months). The lesions demonstrated the spectrum of histologic features described in PVL, and in some cases, patients developed oral cavity squamous cell carcinoma (SCC). An expert working group of oral and maxillofacial and head and neck pathologists reviewed microscopic features in a rigorous fashion, in combination with review of clinical photographs when available. The working group then selected 43 single slide biopsy cases for whole slide digital imaging (WSI) review by members of the consensus conference. The digital images were then reviewed in two surveys separated by a washout period of at least 90 days. Five non-PVL histologic mimics were included as controls. Cases were re-evaluated during a consensus conference with 19 members reporting on the cases. The best inter-observer diagnostic agreement relative to PVL lesions were classified as "corrugated ortho(para)hyperkeratotic lesion, not reactive" and "SCC" (chi-square p = 0.015). There was less than moderate agreement (kappa < 0.60) for lesions in the "Bulky hyperkeratotic epithelial proliferation, not reactive" category. There was ≥ moderate agreement (> 0.41 kappa) for 35 of 48 cases. This expert consensus guideline has been developed with support and endorsement from the leadership of the American Academy of Oral and Maxillofacial Pathology and the North American Society of Head and Neck Pathologists to recommend the use of standardized histopathologic criteria and descriptive terminology to indicate three categories of lesions within PVL: (1) "corrugated ortho(para)hyperkeratotic lesion, not reactive;" (2) "bulky hyperkeratotic epithelial proliferation, not reactive;" and (3) "suspicious for," or "squamous cell carcinoma." Classification of PVL lesions based on a combination of clinical findings and these histologic descriptive categories is encouraged in order to standardize reporting, aid in future research and potentially guide clinical management.
Keywords: Classification; Consensus; Pathology criteria; Proliferative verrucous leukoplakia; Standardized criteria.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
[Interpretation of "Proliferative verrucous leukoplakia: an expert consensus guideline for stadardized assessment and reporting"].Zhonghua Kou Qiang Yi Xue Za Zhi. 2024 Jul 22;59(8):771-776. doi: 10.3760/cma.j.cn112144-20240116-00026. Online ahead of print. Zhonghua Kou Qiang Yi Xue Za Zhi. 2024. PMID: 39036907 Chinese.
-
Inter-observer Variability in the Diagnosis of Proliferative Verrucous Leukoplakia: Clinical Implications for Oral and Maxillofacial Surgeon Understanding: A Collaborative Pilot Study.Head Neck Pathol. 2020 Mar;14(1):156-165. doi: 10.1007/s12105-019-01035-z. Epub 2019 Apr 10. Head Neck Pathol. 2020. PMID: 30972634 Free PMC article.
-
[Proliferative verrucous leukoplakia. Report of five cases].Mund Kiefer Gesichtschir. 2003 May;7(3):164-70. doi: 10.1007/s10006-003-0472-1. Epub 2003 May 1. Mund Kiefer Gesichtschir. 2003. PMID: 12764683 German.
-
Proliferative verrucous leukoplakia and its related lesions.Oral Oncol. 1999 Jul;35(4):354-9. doi: 10.1016/s1368-8375(99)00007-x. Oral Oncol. 1999. PMID: 10645398 Review.
-
Proliferative verrucous leukoplakia: a proposal for diagnostic criteria.Med Oral Patol Oral Cir Bucal. 2010 Nov 1;15(6):e839-45. Med Oral Patol Oral Cir Bucal. 2010. PMID: 20173704 Review.
Cited by
-
Proceedings of the North American Society of Head and Neck Pathology Companion Meeting, New Orleans, LA, March 12, 2023: Oral Cavity Dysplasia: Why Does Histologic Grading Continue to be Contentious?Head Neck Pathol. 2023 Jun;17(2):292-298. doi: 10.1007/s12105-023-01544-y. Epub 2023 May 15. Head Neck Pathol. 2023. PMID: 37184731 Free PMC article. Review.
-
[Progress in clinicopathological diagnosis of oral potentially malignant disorders].Hua Xi Kou Qiang Yi Xue Za Zhi. 2025 Jun 1;43(3):314-324. doi: 10.7518/hxkq.2025.2024427. Hua Xi Kou Qiang Yi Xue Za Zhi. 2025. PMID: 40523811 Free PMC article. Review. Chinese.
-
Gingival Leukoplakia: Hyperkeratosis with Epithelial Atrophy Is A Frequent Histopathologic Finding.Head Neck Pathol. 2021 Dec;15(4):1235-1245. doi: 10.1007/s12105-021-01333-5. Epub 2021 May 31. Head Neck Pathol. 2021. PMID: 34057694 Free PMC article.
-
The association between skin allergy testing and oral squamous cell carcinoma in oral lichen planus: a retrospective cohort study.Arch Dermatol Res. 2025 Jan 9;317(1):207. doi: 10.1007/s00403-024-03681-y. Arch Dermatol Res. 2025. PMID: 39786644
-
Differences in the landscape of colonized microorganisms in different oral potentially malignant disorders and squamous cell carcinoma: a multi-group comparative study.BMC Microbiol. 2024 Sep 2;24(1):318. doi: 10.1186/s12866-024-03458-3. BMC Microbiol. 2024. PMID: 39223464 Free PMC article.
References
-
- Hansen LS, Olson JA, Silverman S. Proliferative verrucous leukoplakia. A long-term study of thirty patients. Oral Surg Oral Med Oral Pathol. 1985;60(3):285–98. - PubMed
-
- Cabay RJ, Morton TH, Jr, Epstein JB. Proliferative verrucous leukoplakia and its progression to oral carcinoma: a review of the literature. J Oral Pathol Med. 2007;36(5):255–61. - PubMed
-
- Silverman S, Jr, Gorsky M. Proliferative verrucous leukoplakia: a follow-up study of 54 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(2):154–7. - PubMed
-
- Bagan JV, Murillo J, Poveda R, et al. Proliferative verrucous leukoplakia: unusual locations of oral squamous cell carcinomas, and field cancerization as shown by the appearance of multiple OSCCs. Oral Oncol. 2004;40(4):440–3. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

