The distinct roles of myosin IIA and IIB under compression stress in nucleus pulposus cells
- PMID: 33415745
- PMCID: PMC7848961
- DOI: 10.1111/cpr.12987
The distinct roles of myosin IIA and IIB under compression stress in nucleus pulposus cells
Abstract
Objectives: Inappropriate or excessive compression applied to intervertebral disc (IVD) contributes substantially to IVD degeneration. The actomyosin system plays a leading role in responding to mechanical stimuli. In the present study, we investigated the roles of myosin II isoforms in the compression stress-induced senescence of nucleus pulposus (NP) cells.
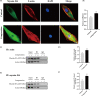
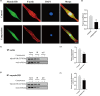
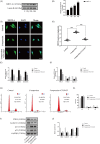
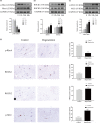
Material and methods: Nucleus pulposus cells were exposed to 1.0 MPa compression for 0, 12, 24 or 36 hours. Immunofluorescence and co-immunoprecipitation analysis were used to measure the interaction of myosin IIA and IIB with actin. Western blot analysis and immunofluorescence staining were used to detect nuclear expression and nuclear localization of MRTF-A. In addition, the expression levels of p-RhoA/RhoA, ROCK1/2 and p-MLC/MLC were measured in human NP cells under compression stress and in degenerative IVD tissues.
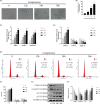
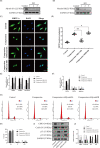
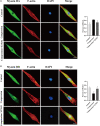
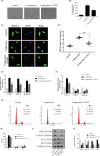
Results: Compression stress increased the interaction of myosin IIA and actin, while the interaction of myosin IIB and actin was reduced. The actomyosin cytoskeleton remodelling was involved in the compression stress-induced fibrotic phenotype mediated by MRTF-A nuclear translocation and inhibition of proliferation in NP cells. Furthermore, RhoA/ROCK1 pathway activation mediated compression stress-induced human NP cells senescence by regulating the interaction of myosin IIA and IIB with actin.
Conclusions: We for the first time investigated the regulation of actomyosin cytoskeleton in human NP cells under compression stress. It provided new insights into the development of therapy for effectively inhibiting IVD degeneration.
Keywords: RhoA/ROCK; actomyosin cytoskeleton; compression stress; intervertebral disc degeneration; myosin II.
© 2021 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures

Similar articles
-
Inhibition of RhoA/MRTF-A signaling alleviates nucleus pulposus fibrosis induced by mechanical stress overload.Connect Tissue Res. 2022 Jan;63(1):53-68. doi: 10.1080/03008207.2021.1952193. Epub 2021 Aug 21. Connect Tissue Res. 2022. PMID: 34420462
-
Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration.Cancer Res. 2006 May 1;66(9):4725-33. doi: 10.1158/0008-5472.CAN-05-4236. Cancer Res. 2006. PMID: 16651425
-
Activity of nonmuscle myosin II isoforms determines localization at the cleavage furrow of megakaryocytes.Blood. 2016 Dec 29;128(26):3137-3145. doi: 10.1182/blood-2016-04-711630. Epub 2016 Oct 13. Blood. 2016. PMID: 27737892
-
Mammalian nonmuscle myosin II comes in three flavors.Biochem Biophys Res Commun. 2018 Nov 25;506(2):394-402. doi: 10.1016/j.bbrc.2018.03.103. Epub 2018 Mar 17. Biochem Biophys Res Commun. 2018. PMID: 29550471 Free PMC article. Review.
-
Rear actomyosin contractility-driven directional cell migration in three-dimensional matrices: a mechano-chemical coupling mechanism.J R Soc Interface. 2014 Mar 19;11(95):20131072. doi: 10.1098/rsif.2013.1072. Print 2014 Jun 6. J R Soc Interface. 2014. PMID: 24647903 Free PMC article. Review.
Cited by
-
Cellular Senescence in Intervertebral Disc Aging and Degeneration: Molecular Mechanisms and Potential Therapeutic Opportunities.Biomolecules. 2023 Apr 18;13(4):686. doi: 10.3390/biom13040686. Biomolecules. 2023. PMID: 37189433 Free PMC article. Review.
-
Scaffold-induced compression enhances ligamentization potential of decellularized tendon graft reseeded with ACL-derived cells.iScience. 2023 Nov 23;26(12):108521. doi: 10.1016/j.isci.2023.108521. eCollection 2023 Dec 15. iScience. 2023. PMID: 38162024 Free PMC article.
-
Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Alleviate Nuclear Pulposus Cells Degeneration Through the miR-145a-5p/USP31/HIF-1α Signaling Pathway.Stem Cell Rev Rep. 2024 Nov;20(8):2268-2282. doi: 10.1007/s12015-024-10781-9. Epub 2024 Aug 30. Stem Cell Rev Rep. 2024. PMID: 39212824
-
Partial reprogramming strategy for intervertebral disc rejuvenation by activating energy switch.Aging Cell. 2022 Apr;21(4):e13577. doi: 10.1111/acel.13577. Epub 2022 Mar 9. Aging Cell. 2022. PMID: 35266272 Free PMC article.
-
Matrix stiffness regulates nucleus pulposus cell glycolysis by MRTF-A-dependent mechanotransduction.Bone Res. 2025 Feb 14;13(1):23. doi: 10.1038/s41413-025-00402-7. Bone Res. 2025. PMID: 39952914 Free PMC article.
References
-
- James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789‐1858. - PMC - PubMed
-
- Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015;84:159‐171. - PubMed
-
- Gullbrand SE, Peterson J, Mastropolo R, et al. Low rate loading‐induced convection enhances net transport into the intervertebral disc in vivo. Spine J. 2015;15(5):1028‐1033. - PubMed
-
- Neidlinger‐Wilke C, Galbusera F, Pratsinis H, et al. Mechanical loading of the intervertebral disc: from the macroscopic to the cellular level. Eur Spine J. 2014;23(suppl 3):S333‐343. - PubMed
MeSH terms
Substances
Grants and funding
- 81772401/National Natural Science Foundation of China
- 81904020/National Natural Science Foundation of China
- U1603121/National Natural Science Foundation of China
- 2019kfyXMBZ06/Fundamental Research Funds for the Central Universities
- 2018YFB1105700/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

