Design of Trehalose-Based Amide/Sulfonamide C-type Lectin Receptor Signaling Compounds
- PMID: 33415819
- PMCID: PMC8068603
- DOI: 10.1002/cmdc.202000775
Design of Trehalose-Based Amide/Sulfonamide C-type Lectin Receptor Signaling Compounds
Erratum in
-
Corrigendum: Design of Trehalose-Based Amide/Sulfonamide C-type Lectin Receptor Signaling Compounds.ChemMedChem. 2022 May 18;17(10):e202100376. doi: 10.1002/cmdc.202100376. Epub 2021 Jun 14. ChemMedChem. 2022. PMID: 34128340 No abstract available.
Abstract
Mincle agonists have been shown to induce inflammatory cytokine production, such as tumor necrosis factor-alpha (TNF) and promote the development of a Th1/Th17 immune response that might be crucial to development of effective vaccination against pathogens such as Mycobacterium tuberculosis. As an expansion of our previous work, a library of 6,6'-amide and sulfonamide α,α-d-trehalose compounds with various substituents on the aromatic ring was synthesized efficiently in good to excellent yields. These compounds were evaluated for their ability to activate the human C-type lectin receptor Mincle by the induction of cytokines from human peripheral blood mononuclear cells. A preliminary structure-activity relationship (SAR) of these novel trehalose diamides and sulfonamides revealed that aryl amide-linked trehalose compounds demonstrated improved activity and relatively high potency cytokine production compared to the Mincle ligand trehalose dibehenate adjuvant (TDB) and the natural ligand trehalose dimycolate (TDM) inducing dose-dependent and human-Mincle-specific stimulation in a HEK reporter cell line.
Keywords: Mincle; TH-17; adjuvants; trehalose tuberculosis; vaccines.
© 2021 Wiley-VCH GmbH.
Figures


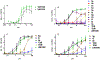

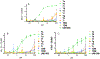
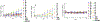
References
-
- Riese P, Schulze K, Ebensen T, Prochnow B, Guzman CA, Current Top. Med. Chem, 2013, 13 (20), 2562–2580. - PubMed
-
- Matsumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, Akira S, J. Immunol, 1999, 163, 5039–5048; - PubMed
- Foster AJ, Nagata M, Lu X, Lynch AT, Omahdi Z, Ishikawa E, Yomasaki S, Timmer MSM, Stocker BL, J. Med. Chem, 2018, 61, 3, 1045–1060; - PubMed
- Matsunaga I, Moody DB, J. Exp. Med, 2009, 206, 2865–2868. - PMC - PubMed
-
- Fomsgaard A, Karlsson I, Gram G, Schou C, Tang S, Bang P, Kromann I, Andersen P, Andreasen LV, Vaccine 2011, 29 , 7067–74; - PubMed
- Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S, Andersen P, Ruland J, Brown GD, Wells C, Lang R, J. Immunol, 2010, 184 , 2756–2760; - PMC - PubMed
- Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S, J. Exp. Med, 2009, 206 , 2879–2888. - PMC - PubMed
-
- Bird JH, Khan AA, Nishimura N, Yamasaki S, Timmer MSM, Stocker BL, J. Org. Chem, 2018, 83, 7593–7605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

