MicroRNA‑34a‑5p expression in the plasma and in its extracellular vesicle fractions in subjects with Parkinson's disease: An exploratory study
- PMID: 33416118
- PMCID: PMC7797475
- DOI: 10.3892/ijmm.2020.4806
MicroRNA‑34a‑5p expression in the plasma and in its extracellular vesicle fractions in subjects with Parkinson's disease: An exploratory study
Abstract
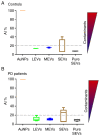
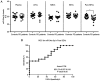
Parkinson's disease (PD) is an important disabling age‑related disorder and is the second most common neurodegenerative disease. Currently, no established molecular biomarkers exist for the early diagnosis of PD. Circulating microRNAs (miRNAs), either vesicle‑free or encapsulated in extracellular vesicles (EVs), have emerged as potential blood‑based biomarkers also for neurodegenerative diseases. In this exploratory study, we focused on miR‑34a‑5p because of its well‑documented involvement in neurobiology. To explore a differential profile of circulating miR‑34a‑5p in PD, PD patients and age‑matched control subjects were enrolled. Serial ultracentrifugation steps and density gradient were used to separate EV subpopulations from plasma according to their different sedimentation properties (Large, Medium, Small EVs). Characterization of EV types was performed using western blotting and atomic force microscopy (AFM); purity from protein contaminants was checked with the colorimetric nanoplasmonic assay. Circulating miR‑34a‑5p levels were evaluated using qPCR in plasma and in each EV type. miR‑34a‑5p was significantly up‑regulated in small EVs devoid of exogenous protein contaminants (pure SEVs) from PD patients and ROC analysis indicated a good diagnostic performance in discriminating patients from controls (AUC=0.74, P<0.05). Moreover, miR‑34a‑5p levels in pure SEVs were associated with disease duration, Hoehn and Yahr and Beck Depression Inventory scores. These results underline the necessity to examine the miRNA content of each EV subpopulation to identify miRNA candidates with potential diagnostic value and lay the basis for future studies to validate the overexpression of circulating miR‑34a‑5p in PD via the use of pure SEVs.
Keywords: Parkinson's disease; human plasma; extracellular vesicles; microRNAs; miR-34a-5p.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

