Novel quinazolinone MJ‑33 induces AKT/mTOR‑mediated autophagy‑associated apoptosis in 5FU‑resistant colorectal cancer cells
- PMID: 33416156
- PMCID: PMC7757098
- DOI: 10.3892/or.2020.7882
Novel quinazolinone MJ‑33 induces AKT/mTOR‑mediated autophagy‑associated apoptosis in 5FU‑resistant colorectal cancer cells
Abstract
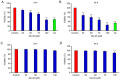
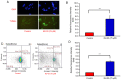
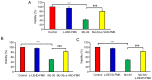
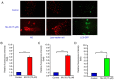
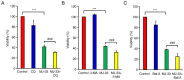
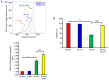
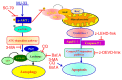
Novel quinazolinone compounds have been studied in the field of drug discovery for a long time. Among their broad range of pharmacological effects, certain compounds effectively inhibit cancer cell proliferation. MJ‑33 is a quinazolinone derivative with proposed anticancer activities that was synthesized in our laboratory. The present study aimed to evaluate the anticancer activity of MJ‑33 in fluorouracil (5FU)‑resistant colorectal cancer cells (HT‑29/5FUR) and to investigate the underlying molecular mechanisms. The cell viability assay results indicated that HT‑29/5FUR cell viability was inhibited by MJ‑33 treatment in a concentration‑dependent manner compared with the control group. The cellular morphological alterations observed following MJ‑33 treatment indicated the occurrence of apoptosis and autophagy, as well as inhibition of cell proliferation in a time‑dependent manner compared with the control group. The acridine orange, LysoTracker Red and LC3‑green fluorescent protein staining results indicated that MJ‑33 treatment significantly induced autophagy compared with the control group. The DAPI/TUNEL dual staining results demonstrated increased nuclear fragmentation and condensation following MJ‑33 treatment compared with the control group. The Annexin V apoptosis assay and image cytometry analysis results demonstrated a significant increase in apoptotic cells following MJ‑33 treatment compared with the control group. The western blotting results demonstrated markedly decreased Bcl‑2, phosphorylated (p)‑BAD, pro‑caspase‑9 and pro‑caspase‑3 expression levels, and notably increased cytochrome c and apoptotic peptidase activating factor 1 expression levels following MJ‑33 treatment compared with the control group. Moreover, the expression levels of autophagy‑related proteins, including autophagy related (ATG)‑5, ATG‑7, ATG‑12, ATG‑16, p62 and LC3‑II, were increased following MJ‑33 treatment compared with the control group. Furthermore, MJ‑33‑treated HT‑29/5FUR cells displayed decreased expression levels of p‑AKT and p‑mTOR compared with control cells. The results suggested that MJ‑33‑induced apoptosis was mediated by AKT signaling, and subsequently modulated via the mitochondria‑dependent signaling pathway. Therefore, the results suggested that suppression of AKT/mTOR activity triggered autophagy in the HT‑29/5FUR cell line. In summary, the results indicated that MJ‑33 inhibited HT‑29/5FUR cell viability, and induced apoptosis and autophagy via the AKT/mTOR signaling pathway. The present study may provide novel insight into the anticancer effects and mechanisms underlying MJ‑33 in 5FU‑resistant colorectal cancer cells.
Keywords: MJ‑33; colorectal cancer; fluorouracil‑resistant HT‑29 cells; autophagy; apoptosis.
Figures



Similar articles
-
RA-XII, a bicyclic hexapeptidic glucoside isolated from Rubia yunnanensis Diels, exerts antitumor activity by inhibiting protective autophagy and activating Akt-mTOR pathway in colorectal cancer cells.J Ethnopharmacol. 2021 Feb 10;266:113438. doi: 10.1016/j.jep.2020.113438. Epub 2020 Oct 2. J Ethnopharmacol. 2021. PMID: 33017635
-
Novel PI3K/Akt/mTOR signaling inhibitor, W922, prevents colorectal cancer growth via the regulation of autophagy.Int J Oncol. 2021 Jan;58(1):70-82. doi: 10.3892/ijo.2020.5151. Epub 2020 Nov 23. Int J Oncol. 2021. PMID: 33367926 Free PMC article.
-
Gossypin induces apoptosis and autophagy via the MAPK/JNK pathway in HT‑29 human colorectal cancer cells.Int J Mol Med. 2025 Jul;56(1):107. doi: 10.3892/ijmm.2025.5548. Epub 2025 May 16. Int J Mol Med. 2025. PMID: 40376978 Free PMC article.
-
Rational targeting of autophagy in colorectal cancer therapy: From molecular interactions to pharmacological compounds.Environ Res. 2023 Jun 15;227:115721. doi: 10.1016/j.envres.2023.115721. Epub 2023 Mar 23. Environ Res. 2023. PMID: 36965788 Review.
-
Different Roles of Apoptosis and Autophagy in the Development of Human Colorectal Cancer.Int J Mol Sci. 2023 Jun 15;24(12):10201. doi: 10.3390/ijms241210201. Int J Mol Sci. 2023. PMID: 37373349 Free PMC article. Review.
Cited by
-
Autophagy Modulators in Cancer Therapy.Int J Mol Sci. 2021 May 28;22(11):5804. doi: 10.3390/ijms22115804. Int J Mol Sci. 2021. PMID: 34071600 Free PMC article. Review.
-
Bioinformatics Analysis and Experimental Validation of Epigallocatechin-3-gallate Against Iopromide-induced Injury in HEK-293 Cells via Anti-oxidative and Anti-inflammation Pathways.In Vivo. 2024 Nov-Dec;38(6):2617-2628. doi: 10.21873/invivo.13738. In Vivo. 2024. PMID: 39477405 Free PMC article.
-
High Concentration of Iopromide Induces Apoptosis and Autophagy in Human Embryonic Kidney Cells via Activating a ROS-dependent Cellular Stress Pathway.In Vivo. 2021 Nov-Dec;35(6):3221-3232. doi: 10.21873/invivo.12617. In Vivo. 2021. PMID: 34697153 Free PMC article.
-
Metformin induces autophagy of cisplatin-resistant human gastric cancer cells in addition to apoptosis.Biomedicine (Taipei). 2023 Jun 1;13(2):14-23. doi: 10.37796/2211-8039.1408. eCollection 2023. Biomedicine (Taipei). 2023. PMID: 37937302 Free PMC article.
-
Protective effects of Jing-Si-herbal-tea in inflammatory cytokines-induced cell injury on normal human lung fibroblast via multiomic platform analysis.Tzu Chi Med J. 2024 Mar 26;36(2):152-165. doi: 10.4103/tcmj.tcmj_267_23. eCollection 2024 Apr-Jun. Tzu Chi Med J. 2024. PMID: 38645788 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

