Targeting translesion synthesis (TLS) to expose replication gaps, a unique cancer vulnerability
- PMID: 33416413
- PMCID: PMC7837368
- DOI: 10.1080/14728222.2021.1864321
Targeting translesion synthesis (TLS) to expose replication gaps, a unique cancer vulnerability
Abstract
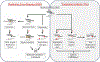
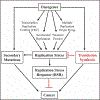
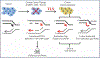
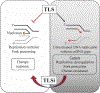
Introduction: Translesion synthesis (TLS) is a DNA damage tolerance (DDT) mechanism that employs error-prone polymerases to bypass replication blocking DNA lesions, contributing to a gain in mutagenesis and chemo-resistance. However, recent findings illustrate an emerging role for TLS in replication gap suppression (RGS), distinct from its role in post-replication gap filling. Here, TLS protects cells from replication stress (RS)-induced toxic single-stranded DNA (ssDNA) gaps that accumulate in the wake of active replication. Intriguingly, TLS-mediated RGS is specifically observed in several cancer cell lines and contributes to their survival. Thus, targeting TLS has the potential to uniquely eradicate tumors without harming non-cancer tissues. Areas Covered: This review provides an innovative perspective on the role of TLS beyond its canonical function of lesion bypass or post-replicative gap filling. We provide a comprehensive analysis that underscores the emerging role of TLS as a cancer adaptation necessary to overcome the replication stress response (RSR), an anti-cancer barrier. Expert Opinion: TLS RGS is critical for tumorigenesis and is a new hallmark of cancer. Although the exact mechanism and extent of TLS dependency in cancer is still emerging, TLS inhibitors have shown promise as an anti-cancer therapy in selectively targeting this unique cancer vulnerability.
Keywords: DNA lesion bypass; Translesion synthesis (TLS); cancer and cancer therapeutics; mutagenesis; oncogene-induced replication stress; replication gap suppression (RGS); replication stress response (RSR); ssDNA gaps.
Conflict of interest statement
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
References
-
- Hejna JA, Moses RE, in Encyclopedia of Microbiology (Third Edition), Schaechter M, Ed. (Academic Press, Oxford, 2009), pp. 113–122.
-
- Samson RY, Bell SD, in The Enzymes, Kaguni LS, Oliveira MT, Eds. (Academic Press, 2016), vol. 39, pp. 169–190. - PubMed
-
- Hoeijmakers JH, DNA damage, aging, and cancer. N Engl J Med 361, 1475–1485 (2009). - PubMed
-
- Mazouzi A, Velimezi G, Loizou JI, DNA replication stress: causes, resolution and disease. Exp Cell Res 329, 85–93 (2014). - PubMed
-
-
Zeman MK, Cimprich KA, Causes and consequences of replication stress. Nat Cell Biol 16, 2–9 (2014).
*are of importance as they provide a comprehensive review on replication stress and TLS pathway.
-
Reference Annotations
-
- ** = The following references (67, 71, 74, 122, 123, 133, 134 and 135) are of considerable importance as they provide supporting evidence for the role of TLS in RGS and in overcoming oncogene induced stress and illustrate the therapeutic potential of targeting TLS in cancer.
-
- * = The following references (5, 24, 46, 52 and 73) are of importance as they provide a comprehensive review on replication stress and TLS pathway.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
