Run for your life: an integrated virtual tissue platform for incorporating exercise oncology into immunotherapy
- PMID: 33416943
- PMCID: PMC10991577
- DOI: 10.1007/s00262-020-02790-7
Run for your life: an integrated virtual tissue platform for incorporating exercise oncology into immunotherapy
Abstract
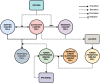
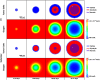
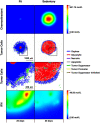
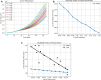
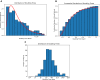
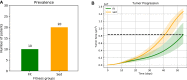
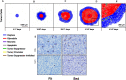
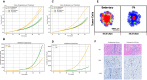
The purpose of this paper is to introduce a novel in silico platform for simulating early-stage solid tumor growth and anti-tumor immune response. We present the model, test the sensitivity and robustness of its parameters, and calibrate it with clinical data from exercise oncology experiments which offer a natural biological backdrop for modulation of anti-tumor immune response. We then perform two virtual experiments with the model that demonstrate its usefulness in guiding pre-clinical and clinical studies of immunotherapy. The first virtual experiment describes the intricate dynamics in the tumor microenvironment between the tumor and the infiltrating immune cells. Such dynamics is difficult to probe during a pre-clinical study as it requires significant redundancy in lab animals and is prohibitively time-consuming and labor-intensive. The result is a series of spatiotemporal snapshots of the tumor and its microenvironment that can serve as a platform to test mechanistic hypotheses on the role and dynamics of different immune cells in anti-tumor immune response. The second virtual experiment shows how dosage and/or frequency of immunotherapy drugs can be optimized based on the aerobic fitness of the patient, so that possible adverse side effects of the treatment can be minimized.
Keywords: Exercise oncology; Glycolysis; Hypoxia; In silico modeling; Personalized medicine; Treg recruitment.
Conflict of interest statement
AH is the inventor and owner of US patent 10.497.467.B2 Systems and Methods for Optimizing Diagnostics and Therapeutics with Metabolic Profiling, and the founder of Cellsor LLC, an exercise oncology start up company.
Figures
Similar articles
-
Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers.J Immunother Cancer. 2018 Jul 3;6(1):65. doi: 10.1186/s40425-018-0376-0. J Immunother Cancer. 2018. PMID: 29970158 Free PMC article. Review.
-
Expression of costimulatory and inhibitory receptors in FoxP3+ regulatory T cells within the tumor microenvironment: Implications for combination immunotherapy approaches.Adv Cancer Res. 2019;144:193-261. doi: 10.1016/bs.acr.2019.05.001. Epub 2019 Jun 6. Adv Cancer Res. 2019. PMID: 31349899 Review.
-
Checkpoint blockade-based immunotherapy in the context of tumor microenvironment: Opportunities and challenges.Cancer Med. 2018 Sep;7(9):4517-4529. doi: 10.1002/cam4.1722. Epub 2018 Aug 7. Cancer Med. 2018. PMID: 30088347 Free PMC article. Review.
-
CIMT 2017: Anniversary symposium - Report on the 15th CIMT Annual Meeting of the Association for Cancer Immunotherapy.Hum Vaccin Immunother. 2017 Oct 3;13(10):2272-2279. doi: 10.1080/21645515.2017.1358327. Hum Vaccin Immunother. 2017. PMID: 28846471 Free PMC article. No abstract available.
-
Immunomodulatory effects of current cancer treatment and the consequences for follow-up immunotherapeutics.Future Oncol. 2017 Aug;13(18):1649-1663. doi: 10.2217/fon-2017-0117. Epub 2017 Aug 4. Future Oncol. 2017. PMID: 28776423 Review.
Cited by
-
Is There a Role for Exercise When Treating Patients with Cancer with Immune Checkpoint Inhibitors? A Scoping Review.Cancers (Basel). 2022 Oct 14;14(20):5039. doi: 10.3390/cancers14205039. Cancers (Basel). 2022. PMID: 36291823 Free PMC article.
References
-
- Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res. 2016;76(14):4032–4050. doi: 10.1158/0008-5472.CAN-16-0887. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

