Rituximab for the treatment of multiple sclerosis: a review
- PMID: 33416999
- PMCID: PMC7790722
- DOI: 10.1007/s00415-020-10362-z
Rituximab for the treatment of multiple sclerosis: a review
Abstract
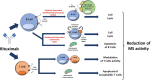
In the last decades, evidence suggesting the direct or indirect involvement of B cells on multiple sclerosis (MS) pathogenesis has accumulated. The increased amount of data on the efficacy and safety of B-cell-depleting therapies from several studies has suggested the addition of these drugs as treatment options to the current armamentarium of disease modifying therapies (DMTs) for MS. Particularly, rituximab (RTX), a chimeric monoclonal antibody directed at CD20 positive B lymphocytes resulting in cell-mediated apoptosis, has been demonstrated to reduce inflammatory activity, incidence of relapses and new brain lesions on magnetic resonance imaging (MRI) in patients with relapsing-remitting MS (RRMS). Additional evidence also demonstrated that patients with progressive MS (PMS) may benefit from RTX, which also showed to be well tolerated, with acceptable safety risks and favorable cost-effectiveness profile.Despite these encouraging results, RTX is currently approved for non-Hodgkin's lymphoma, chronic lymphocytic leukemia, several forms of vasculitis and rheumatoid arthritis, while it can only be administered off-label for MS treatment. Between Northern European countries exist different rules for using not licensed drug for treating MS. The Sweden MS register reports a high rate (53.5%) of off-label RTX prescriptions in relation to other annually started DMTs to treat MS patients, while Danish and Norwegian neurologists have to use other anti-CD20 drugs, as ocrelizumab, in most of the cases.In this paper, we review the pharmacokinetics, pharmacodynamics, clinical efficacy, safety profile and cost effectiveness aspects of RTX for the treatment of MS. Particularly, with the approval of new anti-CD20 DMTs, the recent worldwide COVID-19 emergency and the possible increased risk of infection with this class of drugs, this review sheds light on the use of RTX as an alternative treatment option for MS management, while commenting the gaps of knowledge regarding this drug.
Keywords: Efficacy; Multiple sclerosis; Rituximab; Safety.
© 2021. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Clara Grazia Chisari received grant for congress participation from Almiral, Biogen, Merck Serono, Novartis, Roche, Sanofi and TEVA. Eleonora Sgarlata declares no conflict of interest. Sebastiano Arena declares no conflict of interest. Simona Toscano declares no conflict of interest. Maria Luca declares no conflict of interest. Francesco Patti has received honoraria for speaking activities by Bayer Schering, Biogen, Merck Serono, Novartis and Sanofi Aventis; he also served as advisory board member the following companies: Bayer Schering, Biogen Idec, Merck Serono, Novartis; he was also funded by Pfizer and FISM for epidemiological studies; finally he received grant for congress participation from Bayer Schering, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi Aventis and TEVA.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical