Multidrug resistance-associated protein 4 (Mrp4) is a novel genetic factor in the pathogenesis of obesity and diabetes
- PMID: 33417247
- PMCID: PMC8628361
- DOI: 10.1096/fj.202001299RR
Multidrug resistance-associated protein 4 (Mrp4) is a novel genetic factor in the pathogenesis of obesity and diabetes
Abstract
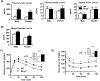
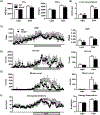
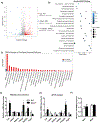
Multidrug resistance protein 4 (Mrp4) is an efflux transporter known to transport several xenobiotics and endogenous molecules. We recently identified that the lack of Mrp4 increases adipose tissue and body weights in mice. However, the role of Mrp4 in adipose tissue physiology are unknown. The current study aimed at characterizing these specific roles of Mrp4 using wild-type (WT) and knockout (Mrp4-/- ) mice. Our studies determined that Mrp4 is expressed in mouse adipose tissue and that the lack of Mrp4 expression is associated with adipocyte hypertrophy. Furthermore, the lack of Mrp4 increased blood glucose and leptin levels, and impaired glucose tolerance. Additionally, in 3T3-L1 cells and human pre-adipocytes, pharmacological inhibition of Mrp4 increased adipogenesis and altered expression of adipogenic genes. Lack of Mrp4 activity in both of our in vivo and in vitro models leads to increased activation of adipose tissue cAMP response element-binding protein (Creb) and decreased plasma prostaglandin E (PGE) metabolite levels. These changes in Creb activation, coupled with decreased PGE levels, together promoted the observed metabolic phenotype in Mrp4-/- mice. In conclusion, our results indicate that Mrp4 as a novel genetic factor involved in the pathogenesis of metabolic diseases, such as obesity and diabetes.
Keywords: Adipogenesis; Drug transporters; Mrp4.
© 2021 Federation of American Societies for Experimental Biology.
Figures
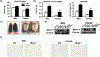

Similar articles
-
MRP4 deficiency drives lipid metabolism dysregulation and adipose tissue inflammation through cAMP-CREB-CRTC2 activation.Life Sci. 2025 Aug 13:123895. doi: 10.1016/j.lfs.2025.123895. Online ahead of print. Life Sci. 2025. PMID: 40816505
-
Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity.Diabetologia. 2018 Feb;61(2):369-380. doi: 10.1007/s00125-017-4471-4. Epub 2017 Oct 24. Diabetologia. 2018. PMID: 29067487 Free PMC article.
-
Loss of G protein pathway suppressor 2 in human adipocytes triggers lipid remodeling by upregulating ATP binding cassette subfamily G member 1.Mol Metab. 2020 Dec;42:101066. doi: 10.1016/j.molmet.2020.101066. Epub 2020 Aug 13. Mol Metab. 2020. PMID: 32798719 Free PMC article.
-
Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds.Mol Biol Rep. 2021 Jan;48(1):743-761. doi: 10.1007/s11033-020-06036-8. Epub 2020 Dec 4. Mol Biol Rep. 2021. PMID: 33275195 Review.
-
Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids.J Intern Med. 2016 Nov;280(5):465-475. doi: 10.1111/joim.12540. Epub 2016 Oct 3. J Intern Med. 2016. PMID: 27699898 Free PMC article. Review.
Cited by
-
Progress and challenges of multidrug resistance proteins in diseases.Am J Cancer Res. 2022 Oct 15;12(10):4483-4501. eCollection 2022. Am J Cancer Res. 2022. PMID: 36381332 Free PMC article. Review.
-
Obesity-related drug transporter expression alterations in human liver and kidneys.Pharmacol Rep. 2024 Dec;76(6):1429-1442. doi: 10.1007/s43440-024-00665-7. Epub 2024 Oct 16. Pharmacol Rep. 2024. PMID: 39412582 Free PMC article.
-
The Prostaglandin E2 Pathway and Breast Cancer Stem Cells: Evidence of Increased Signaling and Potential Targeting.Front Oncol. 2022 Jan 19;11:791696. doi: 10.3389/fonc.2021.791696. eCollection 2021. Front Oncol. 2022. PMID: 35127497 Free PMC article. Review.
-
Investigating the crosstalk between ABCC4 and ABCC5 in 3T3-L1 adipocyte differentiation.Front Mol Biosci. 2024 Dec 9;11:1498946. doi: 10.3389/fmolb.2024.1498946. eCollection 2024. Front Mol Biosci. 2024. PMID: 39717760 Free PMC article.
-
Bioinformatics prediction and experimental verification of key biomarkers for diabetic kidney disease based on transcriptome sequencing in mice.PeerJ. 2022 Sep 20;10:e13932. doi: 10.7717/peerj.13932. eCollection 2022. PeerJ. 2022. PMID: 36157062 Free PMC article.
References
-
- https://www.cdc.gov/diabetes/data/index.html. Vol. 2017. p. CDC Prevalence of Diabetes
-
- https://www.cdc.gov/obesity/data/adult.html. Vol. 2017. p. CDC Obesity prevalence statistics
-
- Ortega FB, Lavie CJ, and Blair SN (2016) Obesity and Cardiovascular Disease. Circulation research 118, 1752–1770 - PubMed
-
- Casanueva FF, Moreno B, Rodriguez-Azeredo R, Massien C, Conthe P, Formiguera X, Barrios V, and Balkau B (2010) Relationship of abdominal obesity with cardiovascular disease, diabetes and hyperlipidaemia in Spain. Clinical endocrinology 73, 35–40 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

