PRSS55 plays an important role in the structural differentiation and energy metabolism of sperm and is required for male fertility in mice
- PMID: 33417308
- PMCID: PMC7882947
- DOI: 10.1111/jcmm.16116
PRSS55 plays an important role in the structural differentiation and energy metabolism of sperm and is required for male fertility in mice
Abstract
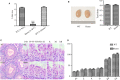
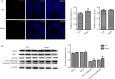
Orderly and stage-specifically expressed proteins are essential for spermatogenesis, and proteases play a key role in protein activation and function. The present study aimed to investigate serine protease 55 (PRSS55), which was reported to play a role in sperm-uterotubal junction (UTJ) migration and sperm-zona pellucida (ZP) binding. We found that PRSS55 was specifically expressed in testicular spermatids and epididymal spermatozoa. By constructing knockout mice targeting all transcripts of Prss55, we demonstrated that deletion of Prss55 resulted in a serious decline of male fertility, with significantly increased sperm malformation and decreased sperm motility. In Prss55-/- mice, increased structural abnormality, including deficient "9 + 2" microtubules, damaged peripheral dense fibre, and defective mitochondrial cristae, were found in sperm. In addition, sperm showed decreased expression of electron transfer chain molecules and lower ATP contents. These could be the potential causes of the astheno/teratozoospermia phenotype of the Prss55-/- mice, and provided new evidence for the previously reported impaired sperm-UTJ migration. Moreover, preliminary studies allowed us to speculate that PRSS55 might function by activating type II muscle myosin in the testis, which is involved in many processes requiring motivation and cytoskeleton translocation. Thus, PRSS55 is essential for the structural differentiation and energy metabolism of sperm, and might be a potential pathogenic factor in astheno/teratozoospermia. Our results provide an additional explanation for the male sterility of Prss55-/- mice, and further reveal the role of PRSS55.
Keywords: PRSS55; energy metabolism; male infertility; sperm motility; spermiogenesis.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures






Similar articles
-
Prss55 but not Prss51 is required for male fertility in mice†.Biol Reprod. 2020 Aug 4;103(2):223-234. doi: 10.1093/biolre/ioaa041. Biol Reprod. 2020. PMID: 32301961 Free PMC article.
-
Serine protease PRSS55 is crucial for male mouse fertility via affecting sperm migration and sperm-egg binding.Cell Mol Life Sci. 2018 Dec;75(23):4371-4384. doi: 10.1007/s00018-018-2878-9. Epub 2018 Jul 21. Cell Mol Life Sci. 2018. PMID: 30032357 Free PMC article.
-
Tmprss12 is required for sperm motility and uterotubal junction migration in mice†.Biol Reprod. 2020 Aug 4;103(2):254-263. doi: 10.1093/biolre/ioaa060. Biol Reprod. 2020. PMID: 32529245 Free PMC article.
-
Haploid male germ cells-the Grand Central Station of protein transport.Hum Reprod Update. 2020 Jun 18;26(4):474-500. doi: 10.1093/humupd/dmaa004. Hum Reprod Update. 2020. PMID: 32318721 Review.
-
An update of the regulatory factors of sperm migration from the uterus into the oviduct by genetically manipulated mice.Mol Reprod Dev. 2019 Aug;86(8):935-955. doi: 10.1002/mrd.23180. Epub 2019 May 27. Mol Reprod Dev. 2019. PMID: 31131960 Review.
Cited by
-
A gene deriving from the ancestral sex chromosomes was lost from the X and retained on the Y chromosome in eutherian mammals.BMC Biol. 2022 Jun 9;20(1):133. doi: 10.1186/s12915-022-01338-8. BMC Biol. 2022. PMID: 35676717 Free PMC article.
-
Global phosphoproteomic analysis identified key kinases regulating male meiosis in mouse.Cell Mol Life Sci. 2022 Aug 5;79(8):467. doi: 10.1007/s00018-022-04507-8. Cell Mol Life Sci. 2022. PMID: 35930080 Free PMC article.
-
Identification of reproductive performance in Bali-polled bulls using computer-assisted semen analysis and plasma seminal proteomics.Vet World. 2025 Jan;18(1):102-109. doi: 10.14202/vetworld.2025.102-109. Epub 2025 Jan 14. Vet World. 2025. PMID: 40041504 Free PMC article.
-
PRSS50-mediated inhibition of MKP3/ERK signaling is crucial for meiotic progression and sperm quality.Zool Res. 2024 Sep 18;45(5):1037-1047. doi: 10.24272/j.issn.2095-8137.2023.388. Zool Res. 2024. PMID: 39147718 Free PMC article.
-
TMPRSS12 Functions in Meiosis and Spermiogenesis and Is Required for Male Fertility in Mice.Front Cell Dev Biol. 2022 Apr 25;10:757042. doi: 10.3389/fcell.2022.757042. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35547804 Free PMC article.
References
-
- Kobori Y. Home testing for male factor infertility: a review of current options. Fertil Steril. 2019;111:864‐870. - PubMed
-
- Kierszenbaum AL, Rivkin E, Tres LL. Molecular biology of sperm head shaping. Soc Reprod Fertil. 2007;65:33‐43. - PubMed
-
- Verdoes M, Verhelst SHL. Detection of protease activity in cells and animals. Biochim Biophys Acta. 2016;1864(1):130‐142. - PubMed
-
- Zhou C, Kang W, Baba T. Functional characterization of double‐knockout mouse sperm lacking SPAM1 and ACR or SPAM1 and PRSS21 in fertilization. J Reprod Dev. 2012;58(3):330‐337. - PubMed
-
- Cesari A, Monclus Mde L, Tejón GP, Clementi M, Fornes MW. Regulated serine proteinase lytic system on mammalian sperm surface: there must be a role. Theriogenology. 2010;74(5):699‐711.e5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

