Expression of the ace operon in Escherichia coli is triggered in response to growth rate-dependent flux-signal of ATP
- PMID: 33417680
- PMCID: PMC8023577
- DOI: 10.1093/femsle/fnaa221
Expression of the ace operon in Escherichia coli is triggered in response to growth rate-dependent flux-signal of ATP
Abstract
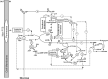
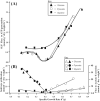
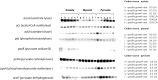
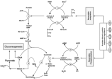
The signal that triggers the expression of the ace operon and, in turn, the transition of central metabolism's architecture from acetogenic to gluconeogenic in Escherichia coli remains elusive despite extensive research both in vivo and in vitro. Here, with the aid of flux analysis together with measurements of the enzymic activity of isocitrate lyase (ICL) and its aceA-messenger ribonucleuc acid (mRNA) transcripts, we provide credible evidence suggesting that the expression of the ace operon in E. coli is triggered in response to growth rate-dependent threshold flux-signal of adenosine triphosphate (ATP). Flux analysis revealed that the shortfall in ATP supply observed as the growth rate ($\mu $) diminishes from µmax to ≤ 0.43h-1 ($ \pm 0.02;n4)\ $is partially redressed by up-regulating flux through succinyl CoA synthetase. Unlike glycerol and glucose, pyruvate cannot feed directly into the two glycolytic ATP-generating reactions catalyzed by phosphoglycerokinase and pyruvate kinase. On the other hand, glycerol, which upon its conversion to D-glyceraldehyde, feeds into the phosphorylation and dephosphorylation parts of glycolysis including the substrate-level phosphorylation-ATP generating reactions, thus preventing ATP flux from dropping to the critical threshold signal required to trigger the acetate-diauxic switch until glycerol is fully consumed. The mRNA transcriptional patterns of key gluconeogenic enzymes, namely, ackA, acetate kinase; pta, phosphotransacetylase; acs, acetyl CoA synthetase and aceA, ICL, suggest that the pyruvate phenotype is better equipped than the glycerol phenotype for the switch from acetogenic to gluconeogenic metabolism.
Keywords: ace operon; acetate-diauxic switch; acetogenic metabolism; flux signals; gluconeogenic metabolism; succinyl CoA synthetase.
© The Author(s) 2021. Published by Oxford University Press on behalf of FEMS.
Figures

Similar articles
-
Coordinated expression of acetyl CoA synthetase and the ace operon enzymes in Escherichia coli in preparation for adaptation to acetate.Microbiology (Reading). 2022 Sep;168(9):001230. doi: 10.1099/mic.0.001230. Microbiology (Reading). 2022. PMID: 36048631 Free PMC article.
-
Contrasting effects of isocitrate dehydrogenase deletion on fluxes through enzymes of central metabolism in Escherichia coli.FEMS Microbiol Lett. 2019 Aug 1;366(15):fnz187. doi: 10.1093/femsle/fnz187. FEMS Microbiol Lett. 2019. PMID: 31504493
-
Physiological and transcriptional comparison of acetate catabolism between Acinetobacter schindleri ACE and Escherichia coli JM101.FEMS Microbiol Lett. 2019 Jun 1;366(12):fnz151. doi: 10.1093/femsle/fnz151. FEMS Microbiol Lett. 2019. PMID: 31281927
-
Control of carbon flux through enzymes of central and intermediary metabolism during growth of Escherichia coli on acetate.Curr Opin Microbiol. 2006 Apr;9(2):173-9. doi: 10.1016/j.mib.2006.02.002. Epub 2006 Mar 10. Curr Opin Microbiol. 2006. PMID: 16530464 Review.
-
Control of isocitrate dehydrogenase catalytic activity by protein phosphorylation in Escherichia coli.J Mol Microbiol Biotechnol. 2005;9(3-4):132-46. doi: 10.1159/000089642. J Mol Microbiol Biotechnol. 2005. PMID: 16415587 Review.
Cited by
-
Coordinated expression of acetyl CoA synthetase and the ace operon enzymes in Escherichia coli in preparation for adaptation to acetate.Microbiology (Reading). 2022 Sep;168(9):001230. doi: 10.1099/mic.0.001230. Microbiology (Reading). 2022. PMID: 36048631 Free PMC article.
References
-
- Bennett PM, Holms WH. Reversible inactivation of the isocitrate dehydrogenase of Escherichia coli ML308 during growth on acetate. J Gen Microbiol. 1975;87:37–51. - PubMed
-
- Bergmeyer HU, Mollering G. Acetate determination with preceding indicator reaction. In: Bergmeyer HU (ed). Methods of Enzymatic Analysis, Vol. 3; 2nd edn. New York & London: Academic Press, 1974, 1520–7.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

