The Duration of Protection from Azithromycin Against Malaria, Acute Respiratory, Gastrointestinal, and Skin Infections When Given Alongside Seasonal Malaria Chemoprevention: Secondary Analyses of Data from a Clinical Trial in Houndé, Burkina Faso, and Bougouni, Mali
- PMID: 33417683
- PMCID: PMC8492219
- DOI: 10.1093/cid/ciaa1905
The Duration of Protection from Azithromycin Against Malaria, Acute Respiratory, Gastrointestinal, and Skin Infections When Given Alongside Seasonal Malaria Chemoprevention: Secondary Analyses of Data from a Clinical Trial in Houndé, Burkina Faso, and Bougouni, Mali
Abstract
Background: Mass drug administration (MDA) with azithromycin (AZ) is being considered as a strategy to promote child survival in sub-Saharan Africa, but the mechanism by which AZ reduces mortality is unclear. To better understand the nature and extent of protection provided by AZ, we explored the profile of protection by time since administration, using data from a household-randomized, placebo-controlled trial in Burkina Faso and Mali.
Methods: Between 2014 and 2016, 30 977 children aged 3-59 months received seasonal malaria chemoprevention (SMC) with sulfadoxine-pyrimethamine plus amodiaquine and either AZ or placebo monthly, on 4 occasions each year. Poisson regression with gamma-distributed random effects, accounting for the household randomization and within-individual clustering of illness episodes, was used to compare incidence of prespecified outcomes between SMC+AZ versus SMC+placebo groups in fixed time strata post-treatment. The likelihood ratio test was used to assess evidence for a time-treatment group interaction.
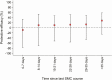
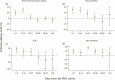
Results: Relative to SMC+placebo, there was no evidence of protection from SMC+AZ against hospital admissions and deaths. Additional protection from SMC+AZ against malaria was confined to the first 2 weeks post-administration (protective efficacy (PE): 24.2% [95% CI: 17.8%, 30.1%]). Gastroenteritis and pneumonia were reduced by 29.9% [21.7; 37.3%], and 34.3% [14.9; 49.3%], respectively, in the first 2 weeks postadministration. Protection against nonmalaria fevers with a skin condition persisted up to 28 days: PE: 46.3% [35.1; 55.6%].
Conclusions: The benefits of AZ-MDA are broad-ranging but short-lived. To maximize impact, timing of AZ-MDA must address the challenge of targeting asynchronous morbidity and mortality peaks from different causes.
Keywords: Azithromycin; Sahel; child mortality; duration of protection; seasonal malaria chemoprevention.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- WHO. WHO guideline on mass drug administration of azithromycin to children under five years of age to promote child survival. Geneva: World Health Organization; 2020. - PubMed
-
- Porco TC, Gebre T, Ayele B, et al. . Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomized trial. JAMA 2009; 302:962–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/R010161/1/European Union
- 205311/Z/16/Z/Wellcome Trust Master's Fellowship in Public Health and Tropical Medicine
- MR/K007319/1/U.K. Medical Research Council, Department for International Development, National Institute for Health Research, and the Wellcome Trust
- MR/K007319/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

