D614G Mutation Alters SARS-CoV-2 Spike Conformation and Enhances Protease Cleavage at the S1/S2 Junction
- PMID: 33417835
- PMCID: PMC7762703
- DOI: 10.1016/j.celrep.2020.108630
D614G Mutation Alters SARS-CoV-2 Spike Conformation and Enhances Protease Cleavage at the S1/S2 Junction
Abstract
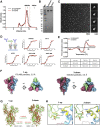
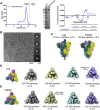
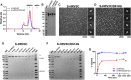
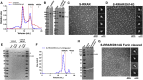
The severe acute respiratory coronavirus 2 (SARS-CoV-2) spike (S) protein is the target of vaccine design efforts to end the coronavirus disease 2019 (COVID-19) pandemic. Despite a low mutation rate, isolates with the D614G substitution in the S protein appeared early during the pandemic and are now the dominant form worldwide. Here, we explore S conformational changes and the effects of the D614G mutation on a soluble S ectodomain construct. Cryoelectron microscopy (cryo-EM) structures reveal altered receptor binding domain (RBD) disposition; antigenicity and proteolysis experiments reveal structural changes and enhanced furin cleavage efficiency of the G614 variant. Furthermore, furin cleavage alters the up/down ratio of the RBDs in the G614 S ectodomain, demonstrating an allosteric effect on RBD positioning triggered by changes in the SD2 region, which harbors residue 614 and the furin cleavage site. Our results elucidate SARS-CoV-2 S conformational landscape and allostery and have implications for vaccine design.
Keywords: 2P; COVID-19; D614G; SARS-CoV-2; allostery; cryo-EM; furin cleavage; spike.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




Update of
-
D614G mutation alters SARS-CoV-2 spike conformational dynamics and protease cleavage susceptibility at the S1/S2 junction.bioRxiv [Preprint]. 2020 Oct 12:2020.10.11.335299. doi: 10.1101/2020.10.11.335299. bioRxiv. 2020. Update in: Cell Rep. 2021 Jan 12;34(2):108630. doi: 10.1016/j.celrep.2020.108630. PMID: 33052347 Free PMC article. Updated. Preprint.
References
-
- Acharya P., Williams W., Henderson R., Janowska K., Manne K., Parks R., Deyton M., Sprenz J., Stalls V., Kopp M. A glycan cluster on the SARS-CoV-2 spike ectodomain is recognized by Fab-dimerized glycan-reactive antibodies. bioRxiv. 2020 doi: 10.1101/2020.06.30.178897. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

