Breast tumor-on-chip models: From disease modeling to personalized drug screening
- PMID: 33417986
- PMCID: PMC8172385
- DOI: 10.1016/j.jconrel.2020.12.057
Breast tumor-on-chip models: From disease modeling to personalized drug screening
Abstract
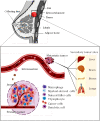
Breast cancer is one of the leading causes of mortality worldwide being the most common cancer among women. Despite the significant progress obtained during the past years in the understanding of breast cancer pathophysiology, women continue to die from it. Novel tools and technologies are needed to develop better diagnostic and therapeutic approaches, and to better understand the molecular and cellular players involved in the progression of this disease. Typical methods employed by the pharmaceutical industry and laboratories to investigate breast cancer etiology and evaluate the efficiency of new therapeutic compounds are still based on traditional tissue culture flasks and animal models, which have certain limitations. Recently, tumor-on-chip technology emerged as a new generation of in vitro disease model to investigate the physiopathology of tumors and predict the efficiency of drugs in a native-like microenvironment. These microfluidic systems reproduce the functional units and composition of human organs and tissues, and importantly, the rheological properties of the native scenario, enabling precise control over fluid flow or local gradients. Herein, we review the most recent works related to breast tumor-on-chip for disease modeling and drug screening applications. Finally, we critically discuss the future applications of this emerging technology in breast cancer therapeutics and drug development.
Keywords: Breast cancer; Drug screening; Industrial applications; Microfluidics; Tumor-on-chip.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
SB, JA and GVC are employers of Elvesys. Elveflow is an Elvesys brand. Authors declare no other conflicts of interest.
Figures




Similar articles
-
Microfluidic Arrays of Breast Tumor Spheroids for Drug Screening and Personalized Cancer Therapies.Adv Healthc Mater. 2022 Jan;11(1):e2101085. doi: 10.1002/adhm.202101085. Epub 2021 Oct 24. Adv Healthc Mater. 2022. PMID: 34636180
-
Breast tumor-on-chip: from the tumor microenvironment to medical applications.Analyst. 2023 Nov 20;148(23):5822-5842. doi: 10.1039/d3an01295f. Analyst. 2023. PMID: 37850340 Review.
-
Metastasis in context: modeling the tumor microenvironment with cancer-on-a-chip approaches.Dis Model Mech. 2018 Mar 16;11(3):dmm033100. doi: 10.1242/dmm.033100. Dis Model Mech. 2018. PMID: 29555848 Free PMC article. Review.
-
Organ-on-a-chip platforms as novel advancements for studying heterogeneity, metastasis, and drug efficacy in breast cancer.Pharmacol Ther. 2022 Sep;237:108156. doi: 10.1016/j.pharmthera.2022.108156. Epub 2022 Feb 10. Pharmacol Ther. 2022. PMID: 35150784 Review.
-
Recent Advances of Organ-on-a-Chip in Cancer Modeling Research.Biosensors (Basel). 2022 Nov 18;12(11):1045. doi: 10.3390/bios12111045. Biosensors (Basel). 2022. PMID: 36421163 Free PMC article. Review.
Cited by
-
Tumor-on-chip platforms for breast cancer continuum concept modeling.Front Bioeng Biotechnol. 2024 Oct 2;12:1436393. doi: 10.3389/fbioe.2024.1436393. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39416279 Free PMC article. Review.
-
Boosting the Clinical Translation of Organ-on-a-Chip Technology.Bioengineering (Basel). 2022 Oct 14;9(10):549. doi: 10.3390/bioengineering9100549. Bioengineering (Basel). 2022. PMID: 36290517 Free PMC article.
-
Mammary Microvessels are Sensitive to Menstrual Cycle Sex Hormones.Adv Sci (Weinh). 2023 Dec;10(35):e2302561. doi: 10.1002/advs.202302561. Epub 2023 Oct 28. Adv Sci (Weinh). 2023. PMID: 37897317 Free PMC article.
-
Stiffness-Controlled Hydrogels for 3D Cell Culture Models.Polymers (Basel). 2022 Dec 17;14(24):5530. doi: 10.3390/polym14245530. Polymers (Basel). 2022. PMID: 36559897 Free PMC article.
-
Organoid models of breast cancer in precision medicine and translational research.Mol Biol Rep. 2024 Nov 21;52(1):2. doi: 10.1007/s11033-024-10101-x. Mol Biol Rep. 2024. PMID: 39570495 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

