Chlorthalidone with potassium citrate decreases calcium oxalate stones and increases bone quality in genetic hypercalciuric stone-forming rats
- PMID: 33417997
- PMCID: PMC8076055
- DOI: 10.1016/j.kint.2020.12.023
Chlorthalidone with potassium citrate decreases calcium oxalate stones and increases bone quality in genetic hypercalciuric stone-forming rats
Abstract
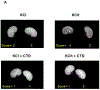
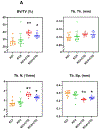
To study human idiopathic hypercalciuria we developed an animal model, genetic hypercalciuric stone-forming rats, whose pathophysiology parallels that of human idiopathic hypercalciuria. Fed the oxalate precursor, hydroxyproline, every rat in this model develops calcium oxalate stones. Using this rat model, we tested whether chlorthalidone and potassium citrate combined would reduce calcium oxalate stone formation and improve bone quality more than either agent alone. These rats (113 generation) were fed a normal calcium and phosphorus diet with hydroxyproline and divided into four groups: diets plus potassium chloride as control, potassium citrate, chlorthalidone plus potassium chloride, or potassium citrate plus chlorthalidone. Urine was collected at six, 12, and 18 weeks and kidney stone formation and bone parameters were determined. Compared to potassium chloride, potassium citrate reduced urinary calcium, chlorthalidone reduced it further and potassium citrate plus chlorthalidone even further. Potassium citrate plus chlorthalidone decreased urine oxalate compared to all other groups. There were no significant differences in calcium oxalate supersaturation in any group. Neither potassium citrate nor chlorthalidone altered stone formation. However, potassium citrate plus chlorthalidone significantly reduced stone formation. Vertebral trabecular bone increased with chlorthalidone and potassium citrate plus chlorthalidone. Cortical bone area increased with chlorthalidone but not potassium citrate or potassium citrate plus chlorthalidone. Mechanical properties of trabecular bone improved with chlorthalidone, but not with potassium citrate plus chlorthalidone. Thus in genetic hypercalciuric stone-forming rats fed a diet resulting in calcium oxalate stone formation, potassium citrate plus chlorthalidone prevented stone formation better than either agent alone. Chlorthalidone alone improved bone quality, but adding potassium citrate provided no additional benefit.
Keywords: calcium oxalate; chlorthalidone; hypercalciuria; nephrolithiasis; potassium citrate.
Copyright © 2021 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Chlorthalidone Is Superior to Potassium Citrate in Reducing Calcium Phosphate Stones and Increasing Bone Quality in Hypercalciuric Stone-Forming Rats.J Am Soc Nephrol. 2019 Jul;30(7):1163-1173. doi: 10.1681/ASN.2018101066. Epub 2019 May 17. J Am Soc Nephrol. 2019. PMID: 31101664 Free PMC article.
-
Effect of Potassium Citrate on Calcium Phosphate Stones in a Model of Hypercalciuria.J Am Soc Nephrol. 2015 Dec;26(12):3001-8. doi: 10.1681/ASN.2014121223. Epub 2015 Apr 8. J Am Soc Nephrol. 2015. PMID: 25855777 Free PMC article.
-
Magnesium Decreases Urine Supersaturation but Not Calcium Oxalate Stone Formation in Genetic Hypercalciuric Stone-Forming Rats.Nephron. 2024;148(7):480-486. doi: 10.1159/000534495. Epub 2024 Jan 23. Nephron. 2024. PMID: 38262368 Free PMC article.
-
Dietary treatment of urinary risk factors for renal stone formation. A review of CLU Working Group.Arch Ital Urol Androl. 2015 Jul 7;87(2):105-20. doi: 10.4081/aiua.2015.2.105. Arch Ital Urol Androl. 2015. PMID: 26150027 Review.
-
Genetic hypercalciuric stone-forming rats.Curr Opin Nephrol Hypertens. 2006 Jul;15(4):403-18. doi: 10.1097/01.mnh.0000232881.35469.a9. Curr Opin Nephrol Hypertens. 2006. PMID: 16775455 Review.
Cited by
-
Sodium glucose co-transporter 2 inhibitor prevents nephrolithiasis in non-diabetes by restoring impaired autophagic flux.EBioMedicine. 2025 Apr;114:105668. doi: 10.1016/j.ebiom.2025.105668. Epub 2025 Mar 25. EBioMedicine. 2025. PMID: 40138887 Free PMC article.
-
Kidney Stone Prevention.Adv Nutr. 2023 May;14(3):555-569. doi: 10.1016/j.advnut.2023.03.002. Epub 2023 Mar 9. Adv Nutr. 2023. PMID: 36906146 Free PMC article. Review.
-
Associations between clinical, biochemical, and nutritional factors in kidney stone formation and recurrence.Urolithiasis. 2025 Jun 12;53(1):112. doi: 10.1007/s00240-025-01784-3. Urolithiasis. 2025. PMID: 40504378
-
Engineered extracellular vesicles as imaging biomarkers and therapeutic applications for urological diseases.Mater Today Bio. 2025 Mar 11;32:101646. doi: 10.1016/j.mtbio.2025.101646. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40160248 Free PMC article. Review.
-
Characterizing Chemokine Signaling Pathways and Hub Genes in Calcium Oxalate-Induced Kidney Stone Formation: Insights from Rodent Models.Biochem Genet. 2025 Feb 1. doi: 10.1007/s10528-025-11036-z. Online ahead of print. Biochem Genet. 2025. PMID: 39893356
References
-
- Bose A, Monk RD, Bushinsky DA. Kidney stones. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (eds). Williams Textbook of Endocrinology, 13 edn. Elsevier: Philadelphia, 2016, pp 1365–1384.
-
- Bushinsky DA, Frick KK, Nehrke K. Genetic hypercalciuric stone-forming rats. Curr Opinion Nephrol Hyperten 2006; 15: 403–418. - PubMed
-
- Bushinsky DA, Parker WR, Asplin JR. Calcium phosphate supersaturation regulates stone formation in genetic hypercalciuric stone-forming rats. Kidney international 2000; 57: 550–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

