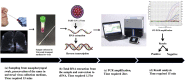
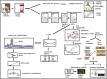
Recent trends in analytical and digital techniques for the detection of the SARS-Cov-2
- PMID: 33418105
- PMCID: PMC7768211
- DOI: 10.1016/j.bpc.2020.106538
Recent trends in analytical and digital techniques for the detection of the SARS-Cov-2
Abstract
The current global outbreak of COVID-19 due to SARS-CoV-2 is an unprecedented humanitarian crisis. Considering the gravity of its impact there is an immediate need to develop a detection technique that is sensitive, specific, fast, and affordable for the clinical diagnosis of the disease. Real time Polymerase Chain Reaction (RT-PCR)-based detection platforms are contemplated to be the gold standard to detect viral RNA. However, that may be susceptible to errors, and there is a risk of obtaining false results, which ultimately compromises the strategy of efficient disease management. Several modern techniques exhibiting assured results with enhanced sensitivity and specificity against the SARS-CoV-2 associated viral components or immune response against it have been developed and may be implemented. The review deals with the conventional RT-PCR detection techniques and compares them to other detection platforms viz., biosensor based detection of antigens, fluorescent or colorimetric detection systems including CRISPR-Cas 13 based SHERLOCK kit, CRISPR Cas-9 based FELUDA test kit, CRISPR DETECTR kit, Next Generation Sequencing or microarray-based kits. These modern techniques are great as a point of care detection methods but should be followed by RT PCR based detection for the confirmation of COVID-19 status.
Keywords: Biosensors; CRISPR-Cas; Immunosensor; Microarray; Next-generation sequencing; Nucleic-acid amplification; RT-PCR; SARS-CoV-2; Serological test.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Rapid, Sensitive, and Specific Severe Acute Respiratory Syndrome Coronavirus 2 Detection: A Multicenter Comparison Between Standard Quantitative Reverse-Transcriptase Polymerase Chain Reaction and CRISPR-Based DETECTR.J Infect Dis. 2021 Feb 3;223(2):206-213. doi: 10.1093/infdis/jiaa641. J Infect Dis. 2021. PMID: 33535237 Free PMC article.
-
Paper-Based Biosensors: Frontiers in Point-of-Care Detection of COVID-19 Disease.Biosensors (Basel). 2021 Apr 7;11(4):110. doi: 10.3390/bios11040110. Biosensors (Basel). 2021. PMID: 33917183 Free PMC article. Review.
-
Label-free Colorimetric Detection of Viral RNA Based on Clustered Regularly Interspaced Short Palindromic Repeats and Gold Nanoparticles with a Portable Device.Langmuir. 2024 Jun 4;40(22):11534-11540. doi: 10.1021/acs.langmuir.4c00657. Epub 2024 May 17. Langmuir. 2024. PMID: 38758706
-
CRISPR is a useful biological tool for detecting nucleic acid of SARS-CoV-2 in human clinical samples.Biomed Pharmacother. 2021 Aug;140:111772. doi: 10.1016/j.biopha.2021.111772. Epub 2021 May 27. Biomed Pharmacother. 2021. PMID: 34062417 Free PMC article. Review.
-
Open Sharing During COVID-19: CRISPR-Based Detection Tools.CRISPR J. 2020 Jun;3(3):142-145. doi: 10.1089/crispr.2020.0030. CRISPR J. 2020. PMID: 33560914 Free PMC article. No abstract available.
Cited by
-
Detection of a characteristic melting profile of a SARS-CoV-2 Kappa variant in Italy using the SARS-CoV-2 Variants ELITe MGB® Kit.J Virol Methods. 2022 Mar;301:114458. doi: 10.1016/j.jviromet.2022.114458. Epub 2022 Jan 10. J Virol Methods. 2022. PMID: 35026304 Free PMC article.
-
Superiority of MALDI-TOF Mass Spectrometry over Real-Time PCR for SARS-CoV-2 RNA Detection.Viruses. 2021 Apr 22;13(5):730. doi: 10.3390/v13050730. Viruses. 2021. PMID: 33922195 Free PMC article.
-
COVID-19 Pandemic: Review of Contemporary and Forthcoming Detection Tools.Infect Drug Resist. 2021 Mar 17;14:1049-1082. doi: 10.2147/IDR.S289629. eCollection 2021. Infect Drug Resist. 2021. PMID: 33762831 Free PMC article. Review.
-
Performance Evaluation of a Novel Ultrafast Molecular Diagnostic Device Integrated With Microfluidic Chips and Dual Temperature Modules.Front Bioeng Biotechnol. 2022 May 19;10:895236. doi: 10.3389/fbioe.2022.895236. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35662850 Free PMC article.
-
Current trends in COVID-19 diagnosis and its new variants in physiological fluids: Surface antigens, antibodies, nucleic acids, and RNA sequencing.Trends Analyt Chem. 2022 Dec;157:116750. doi: 10.1016/j.trac.2022.116750. Epub 2022 Aug 30. Trends Analyt Chem. 2022. PMID: 36060607 Free PMC article. Review.
References
-
- Gorbalenya A.E., Baker S.C., Baric R.S., Groot R.J.D., Drosten C., Gulyaeva A.A., Gulyaeva B.L., Penzar D. Severe acute respiratory syndrome-related coronavirus: The species and its viruses–a statement of the Coronavirus Study Group. bioRxiv. 2020 doi: 10.1101/2020.02.07.937862. - DOI
-
- S.J. Fong, N. Dey and J. Chaki, An introduction to COVID-19, artificial intelligence for coronavirus outbreak. (2021) 1-22. Springer briefs in applied sciences and technology. Springer, Singapore.doi:10.1007/978-981-15-5936-5_1. - DOI
-
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H.…Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. ISSN 0140-6736. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous