Generation of a Novel Mesothelin-Targeted Oncolytic Herpes Virus and Implemented Strategies for Manufacturing
- PMID: 33418877
- PMCID: PMC7825047
- DOI: 10.3390/ijms22020477
Generation of a Novel Mesothelin-Targeted Oncolytic Herpes Virus and Implemented Strategies for Manufacturing
Abstract
Background: HER2-based retargeted viruses are in advanced phases of preclinical development of breast cancer models. Mesothelin (MSLN) is a cell-surface tumor antigen expressed in different subtypes of breast and non-breast cancer. Its recent identification as a marker of some triple-negative breast tumors renders it an attractive target, presently investigated in clinical trials employing antibody drug conjugates and CAR-T cells. The availability of MSLN-retargeted oncolytic viruses may complement the current immunotherapeutic panel of biological drugs against HER2-negative breast and non-breast tumors.
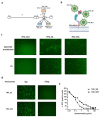
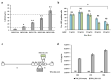
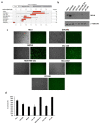
Methods: A fully virulent, tumor-targeted oncolytic Herpes simplex virus-1 (MSLN-THV) with a selectivity for mesothelin-expressing cancer cells was generated. Recombineering technology was used to replace an essential moiety of the viral glycoprotein D with antibody fragments derived from clinically validated MSLN monoclonal antibodies, and to allow IL12 cargo expression in infected cells. Panels of breast and female reproductive system cell lines were used to verify the oncolytic potential of the viral constructs. A platform for production of the retargeted viruses was developed in HEK 293 cells, providing stable expression of a suitable chimeric receptor.
Results: We demonstrated the selectivity of viral infection and cytotoxicity by MSLN-retargeted viruses in a panel of mesothelin-positive cancer cells, originating from breast and female reproductive system tumors. We also developed a second-generation oncolytic MSLN-THV, encoding IL12, to enhance the immunotherapeutic potential of the viral backbone. A non-tumor cell line expressing a chimeric MSLN/Nectin-1 receptor, de-sensitized from antiviral responses by genetic inactivation of the Stimulator of Interferon Genes (STING)-dependent pathway was engineered, to optimize viral yields.
Conclusions: Our proof-of-concept study proposes MSLN-retargeted herpesviruses as potential cancer immunotherapeutics for assessments in preclinical models of MSLN-positive tumors, complementing the available panel of oncolytic viruses to HER2-negative breast tumors.
Keywords: MSLN; malignant mesothelioma; oncolytic virus; targeted therapy; triple negative breast cancer.
Conflict of interest statement
The authors Alfredo Nicosia and Elisa Scarselli are founders and shareholders of Nouscom S.R.L. Emanuele Sasso, Gabriella Cotugno, Anna Morena D’Alise, Armin Lahm are employees of Nouscom S.R.L. All the other authors declare no competing interests.
Figures




Similar articles
-
Enhancing mesothelin CAR T cell therapy for pancreatic cancer with an oncolytic herpes virus boosting CAR target antigen expression.Cancer Immunol Immunother. 2025 May 14;74(7):202. doi: 10.1007/s00262-025-04039-7. Cancer Immunol Immunother. 2025. PMID: 40366419 Free PMC article.
-
A Strategy for Cultivation of Retargeted Oncolytic Herpes Simplex Viruses in Non-cancer Cells.J Virol. 2017 Apr 28;91(10):e00067-17. doi: 10.1128/JVI.00067-17. Print 2017 May 15. J Virol. 2017. PMID: 28250120 Free PMC article.
-
Preclinical therapy of disseminated HER-2⁺ ovarian and breast carcinomas with a HER-2-retargeted oncolytic herpesvirus.PLoS Pathog. 2013 Jan;9(1):e1003155. doi: 10.1371/journal.ppat.1003155. Epub 2013 Jan 31. PLoS Pathog. 2013. PMID: 23382683 Free PMC article.
-
Employing tumor hypoxia for oncolytic therapy in breast cancer.J Mammary Gland Biol Neoplasia. 2005 Oct;10(4):311-8. doi: 10.1007/s10911-006-9004-6. J Mammary Gland Biol Neoplasia. 2005. PMID: 16826462 Review.
-
Mesothelin-targeted CAR-T cell therapy for solid tumors.Expert Opin Biol Ther. 2021 Apr;21(4):473-486. doi: 10.1080/14712598.2021.1843628. Epub 2020 Nov 12. Expert Opin Biol Ther. 2021. PMID: 33176519 Review.
Cited by
-
Generation of a Retargeted Oncolytic Herpes Virus Encoding Adenosine Deaminase for Tumor Adenosine Clearance.Int J Mol Sci. 2021 Dec 16;22(24):13521. doi: 10.3390/ijms222413521. Int J Mol Sci. 2021. PMID: 34948316 Free PMC article.
-
Integrating system biology and intratumor gene therapy by trans-complementing the appropriate co-stimulatory molecule as payload in oncolytic herpes virus.Cancer Gene Ther. 2024 Sep;31(9):1335-1343. doi: 10.1038/s41417-024-00790-8. Epub 2024 Jun 5. Cancer Gene Ther. 2024. PMID: 38839891 Free PMC article.
-
Comparison of the oncolytic activity of a replication-competent and a replication-deficient herpes simplex virus 1.Immunology. 2024 Jun;172(2):279-294. doi: 10.1111/imm.13775. Epub 2024 Mar 5. Immunology. 2024. PMID: 38444199 Free PMC article.
-
The investigation of oncolytic viruses in the field of cancer therapy.Front Oncol. 2024 Jul 10;14:1423143. doi: 10.3389/fonc.2024.1423143. eCollection 2024. Front Oncol. 2024. PMID: 39055561 Free PMC article. Review.
-
Systems Biology Approaches for the Improvement of Oncolytic Virus-Based Immunotherapies.Cancers (Basel). 2023 Feb 17;15(4):1297. doi: 10.3390/cancers15041297. Cancers (Basel). 2023. PMID: 36831638 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

