Cancer and Stress: Does It Make a Difference to the Patient When These Two Challenges Collide?
- PMID: 33418900
- PMCID: PMC7825104
- DOI: 10.3390/cancers13020163
Cancer and Stress: Does It Make a Difference to the Patient When These Two Challenges Collide?
Abstract
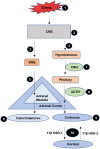
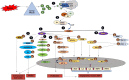
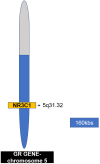
A single head and neck Cancer (HNC) is a globally growing challenge associated with significant morbidity and mortality. The diagnosis itself can affect the patients profoundly let alone the complex and disfiguring treatment. The highly important functions of structures of the head and neck such as mastication, speech, aesthetics, identity and social interactions make a cancer diagnosis in this region even more psychologically traumatic. The emotional distress engendered as a result of functional and social disruption is certain to negatively affect health-related quality of life (HRQoL). The key biological responses to stressful events are moderated through the combined action of two systems, the hypothalamus-pituitary-adrenal axis (HPA) which releases glucocorticoids and the sympathetic nervous system (SNS) which releases catecholamines. In acute stress, these hormones help the body to regain homeostasis; however, in chronic stress their increased levels and activation of their receptors may aid in the progression of cancer. Despite ample evidence on the existence of stress in patients diagnosed with HNC, studies looking at the effect of stress on the progression of disease are scarce, compared to other cancers. This review summarises the challenges associated with HNC that make it stressful and describes how stress signalling aids in the progression of cancer. Growing evidence on the relationship between stress and HNC makes it paramount to focus future research towards a better understanding of stress and its effect on head and neck cancer.
Keywords: HNC; cancer; glucocorticoid signalling; stress; β-adrenergic signalling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress.Dev Neurosci. 1996;18(1-2):49-72. doi: 10.1159/000111395. Dev Neurosci. 1996. PMID: 8840086 Review.
-
The effects of suppressing the biological stress systems on social threat-assessment following acute stress.Psychopharmacology (Berl). 2020 Oct;237(10):3047-3056. doi: 10.1007/s00213-020-05591-z. Epub 2020 Jun 29. Psychopharmacology (Berl). 2020. PMID: 32601985 Free PMC article. Clinical Trial.
-
Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke.Brain Behav Immun. 2014 Oct;41:200-9. doi: 10.1016/j.bbi.2014.05.015. Epub 2014 Jun 2. Brain Behav Immun. 2014. PMID: 24886966
-
Neuroendocrine aspects of the response to stress.Metabolism. 2002 Jun;51(6 Suppl 1):5-10. doi: 10.1053/meta.2002.33184. Metabolism. 2002. PMID: 12040534 Review.
-
[Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context].Encephale. 2012 Oct;38(5):373-80. doi: 10.1016/j.encep.2011.12.003. Epub 2012 Jan 24. Encephale. 2012. PMID: 23062450 Review. French.
Cited by
-
Effects of Serum From Radiofrequency Ablation Patients Receiving General Anesthesia or Local Anesthesia on Hepatocellular Carcinoma Cancer Cell Malignancy: A Prospective Randomized Controlled Trial.Front Oncol. 2021 Sep 23;11:686294. doi: 10.3389/fonc.2021.686294. eCollection 2021. Front Oncol. 2021. PMID: 34631520 Free PMC article.
-
Integrative Multi-Omics Profiling of Rhabdomyosarcoma Subtypes Reveals Distinct Molecular Pathways and Biomarker Signatures.Cells. 2025 Jul 20;14(14):1115. doi: 10.3390/cells14141115. Cells. 2025. PMID: 40710368 Free PMC article.
-
Obesity: The Fat Tissue Disease Version of Cancer.Cells. 2022 Jun 9;11(12):1872. doi: 10.3390/cells11121872. Cells. 2022. PMID: 35741001 Free PMC article. Review.
-
A Transcriptomic Analysis of Head and Neck Squamous Cell Carcinomas for Prognostic Indications.J Pers Med. 2021 Aug 11;11(8):782. doi: 10.3390/jpm11080782. J Pers Med. 2021. PMID: 34442426 Free PMC article.
-
Genetic Contribution of the Adrenergic, Cholinergic, and Serotonergic Systems to Leiomyoma Development and Treatment.Int J Mol Cell Med. 2023;12(4):320-334. doi: 10.22088/IJMCM.BUMS.12.4.320. Int J Mol Cell Med. 2023. PMID: 39006196 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

