Regulation of Complement Activation by Heme Oxygenase-1 (HO-1) in Kidney Injury
- PMID: 33418934
- PMCID: PMC7825075
- DOI: 10.3390/antiox10010060
Regulation of Complement Activation by Heme Oxygenase-1 (HO-1) in Kidney Injury
Abstract
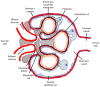
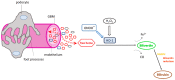
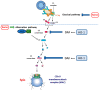
Heme oxygenase is a cytoprotective enzyme with strong antioxidant and anti-apoptotic properties. Its cytoprotective role is mainly attributed to its enzymatic activity, which involves the degradation of heme to biliverdin with simultaneous release of carbon monoxide (CO). Recent studies uncovered a new cytoprotective role for heme oxygenase-1 (HO-1) by identifying a regulatory role on the complement control protein decay-accelerating factor. This is a key complement regulatory protein preventing dysregulation or overactivation of complement cascades that can cause kidney injury. Cell-specific targeting of HO-1 induction may, therefore, be a novel approach to attenuate complement-dependent forms of kidney disease.
Keywords: complement; heme; heme oxygenase-1 (HO-1); kidney injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Heme oxygenase-1 expression enhances vascular endothelial resistance to complement-mediated injury through induction of decay-accelerating factor: a role for increased bilirubin and ferritin.Blood. 2009 Feb 12;113(7):1598-607. doi: 10.1182/blood-2008-04-152934. Epub 2008 Nov 25. Blood. 2009. PMID: 19036700
-
Heme oxygenase and the kidney.DNA Cell Biol. 2002 Apr;21(4):307-21. doi: 10.1089/104454902753759726. DNA Cell Biol. 2002. PMID: 12042070 Review.
-
Therapeutic Potential of Heme Oxygenase-1/carbon Monoxide System Against Ischemia-Reperfusion Injury.Curr Pharm Des. 2017;23(26):3884-3898. doi: 10.2174/1381612823666170413122439. Curr Pharm Des. 2017. PMID: 28412905 Review.
-
Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues.Kidney Int. 2006 Aug;70(3):432-43. doi: 10.1038/sj.ki.5001565. Epub 2006 Jun 14. Kidney Int. 2006. PMID: 16775600 Review.
-
Heme Oxygenase 1 Up-Regulates Glomerular Decay Accelerating Factor Expression and Minimizes Complement Deposition and Injury.Am J Pathol. 2016 Nov;186(11):2833-2845. doi: 10.1016/j.ajpath.2016.07.009. Epub 2016 Sep 21. Am J Pathol. 2016. PMID: 27662796
Cited by
-
Idiopathic inflammatory myopathy and C3 glomerulopathy: a rare association.J Nephrol. 2025 Mar;38(2):733-737. doi: 10.1007/s40620-024-02148-7. Epub 2024 Dec 14. J Nephrol. 2025. PMID: 39674867
-
C3a and C5b-9 Differentially Predict COVID-19 Progression and Outcome.Life (Basel). 2022 Aug 28;12(9):1335. doi: 10.3390/life12091335. Life (Basel). 2022. PMID: 36143371 Free PMC article.
-
The Role of Heme Oxygenase-1 as an Immunomodulator in Kidney Disease.Antioxidants (Basel). 2022 Dec 13;11(12):2454. doi: 10.3390/antiox11122454. Antioxidants (Basel). 2022. PMID: 36552662 Free PMC article. Review.
-
Exploring Potential Complement Modulation Strategies for Ischemia-Reperfusion Injury in Kidney Transplantation.Antioxidants (Basel). 2025 Jan 8;14(1):66. doi: 10.3390/antiox14010066. Antioxidants (Basel). 2025. PMID: 39857400 Free PMC article. Review.
-
Protective Role of Cepharanthine Against Equid Herpesvirus Type 8 Through AMPK and Nrf2/HO-1 Pathway Activation.Viruses. 2024 Nov 12;16(11):1765. doi: 10.3390/v16111765. Viruses. 2024. PMID: 39599879 Free PMC article.
References
-
- Maines M.D. The heme oxygenase system and its functions in the brain. Cell. Mol. Biol. 2000;46:573–585. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

