Inflaming the Brain with Iron
- PMID: 33419006
- PMCID: PMC7825317
- DOI: 10.3390/antiox10010061
Inflaming the Brain with Iron
Abstract
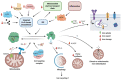
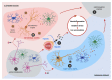
Iron accumulation and neuroinflammation are pathological conditions found in several neurodegenerative diseases, including Alzheimer's disease (AD) and Parkinson's disease (PD). Iron and inflammation are intertwined in a bidirectional relationship, where iron modifies the inflammatory phenotype of microglia and infiltrating macrophages, and in turn, these cells secrete diffusible mediators that reshape neuronal iron homeostasis and regulate iron entry into the brain. Secreted inflammatory mediators include cytokines and reactive oxygen/nitrogen species (ROS/RNS), notably hepcidin and nitric oxide (·NO). Hepcidin is a small cationic peptide with a central role in regulating systemic iron homeostasis. Also present in the cerebrospinal fluid (CSF), hepcidin can reduce iron export from neurons and decreases iron entry through the blood-brain barrier (BBB) by binding to the iron exporter ferroportin 1 (Fpn1). Likewise, ·NO selectively converts cytosolic aconitase (c-aconitase) into the iron regulatory protein 1 (IRP1), which regulates cellular iron homeostasis through its binding to iron response elements (IRE) located in the mRNAs of iron-related proteins. Nitric oxide-activated IRP1 can impair cellular iron homeostasis during neuroinflammation, triggering iron accumulation, especially in the mitochondria, leading to neuronal death. In this review, we will summarize findings that connect neuroinflammation and iron accumulation, which support their causal association in the neurodegenerative processes observed in AD and PD.
Keywords: Alzheimer’s disease; Parkinson’s disease; hepcidin; iron; iron regulatory protein 1; neuroinflammation; nitric oxide; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Theofilas P., Ehrenberg A.J., Dunlop S., Alho A.T.D.L., Nguy A., Leite R.E.P., Rodriguez R.D., Mejia M.B., Suemoto C.K., Ferretti-Rebustini R.E.L., et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 2017;13:236–246. doi: 10.1016/j.jalz.2016.06.2362. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

