Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach
- PMID: 33419065
- PMCID: PMC7825450
- DOI: 10.3390/nu13010159
Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach
Abstract
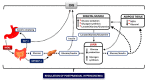
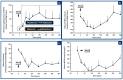
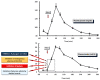
Glucose levels in blood must be constantly maintained within a tight physiological range to sustain anabolism. Insulin regulates glucose homeostasis via its effects on glucose production from the liver and kidneys and glucose disposal in peripheral tissues (mainly skeletal muscle). Blood levels of glucose are regulated simultaneously by insulin-mediated rates of glucose production from the liver (and kidneys) and removal from muscle; adipose tissue is a key partner in this scenario, providing nonesterified fatty acids (NEFA) as an alternative fuel for skeletal muscle and liver when blood glucose levels are depleted. During sleep at night, the gradual development of insulin resistance, due to growth hormone and cortisol surges, ensures that blood glucose levels will be maintained within normal levels by: (a) switching from glucose to NEFA oxidation in muscle; (b) modulating glucose production from the liver/kidneys. After meals, several mechanisms (sequence/composition of meals, gastric emptying/intestinal glucose absorption, gastrointestinal hormones, hyperglycemia mass action effects, insulin/glucagon secretion/action, de novo lipogenesis and glucose disposal) operate in concert for optimal regulation of postprandial glucose fluctuations. The contribution of the liver in postprandial glucose homeostasis is critical. The liver is preferentially used to dispose over 50% of the ingested glucose and restrict the acute increases of glucose and insulin in the bloodstream after meals, thus protecting the circulation and tissues from the adverse effects of marked hyperglycemia and hyperinsulinemia.
Keywords: adipose tissue; fasting; incretins; insulin action secretion; liver; meal sequence; muscle; postabsorptive postprandial glucose metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bender D. Introduction to Nutrition and Metabolism. 2nd ed. Taylor & Francis; London, UK: Bristol, PA, USA: 1997. Why eat? pp. 1–10.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

