Diesel Exhaust Particulates Enhances Susceptibility of LPS-Induced Acute Lung Injury through Upregulation of the IL-17 Cytokine-Derived TGF-β1/Collagen I Expression and Activation of NLRP3 Inflammasome Signaling in Mice
- PMID: 33419073
- PMCID: PMC7825418
- DOI: 10.3390/biom11010067
Diesel Exhaust Particulates Enhances Susceptibility of LPS-Induced Acute Lung Injury through Upregulation of the IL-17 Cytokine-Derived TGF-β1/Collagen I Expression and Activation of NLRP3 Inflammasome Signaling in Mice
Abstract
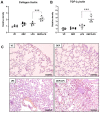
Diesel exhaust particulates (DEP) adversely affect the respiratory system and exacerbate lung diseases, resulting in high mortality rates. However, its pathogenesis is complicated, and the mechanisms involved are incompletely understood. We investigated the effects of DEP pre-exposure on lipopolysaccharide (LPS)-induced acute lung injury (ALI) and identified the roles of interleukin (IL)-17 in mice. Mice were divided into vehicle control, DEP, LPS, and DEP pre-exposed and LPS-instilled groups. Pre-exposure to DEP enhanced the number of total cells, neutrophils, and lymphocytes in the BAL fluid of LPS-instilled mice. Pre-exposure to DEP synergistically exacerbated pulmonary acute lung inflammation and granulomatous inflammation/pulmonary fibrosis, concomitant with the enhanced expression of inflammatory cytokines in the BAL fluid and of collagen I and TGF-β1 in the lungs of LPS-instilled mice. The number of TGF-β1-positive cells in the DEP pre-exposed and LPS-instilled group was higher than that in the LPS group. The expression of NLR family pyrin domain containing 3 (NLRP3) inflammasome components was markedly increased in the DEP pre-exposed and LPS-instilled group. IL-17 levels in the BAL fluid and IL-17-positive cells in the lungs were significantly increased by pre-exposure to DEP in the LPS-induced group compared to that in the DEP or LPS group. These results suggest that DEP predominantly contributes to fibrotic lung disease in LPS-related acute lung injury by upregulating IL-17 cytokine-mediated collagen I and TGF-β1 and, at least in part, by activating LPS-induced NLRP3 inflammasome signaling. The study should be useful in devising better strategies for prevention and management of ALI.
Keywords: IL-17; NLRP3; TGF-β1; acute lung injury; diesel exhaust particulate; lung fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Strosnider H.M., Chang H.H., Darrow L.A., Liu Y., Vaidyanathan A., Strickland M.J. Age-specific associations of ozone and fine particulate matter with respiratory emergency department visits in the United States. Am. J. Respir. Crit. Care Med. 2019;199:882. doi: 10.1164/rccm.201806-1147OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

