Synthesis of Organic Matter in Aqueous Environments Simulating Small Bodies in the Solar System and the Effects of Minerals on Amino Acid Formation
- PMID: 33419105
- PMCID: PMC7825434
- DOI: 10.3390/life11010032
Synthesis of Organic Matter in Aqueous Environments Simulating Small Bodies in the Solar System and the Effects of Minerals on Amino Acid Formation
Abstract
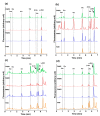
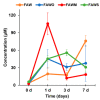
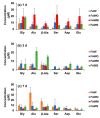
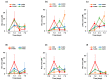
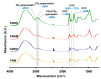
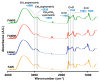
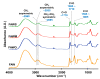
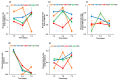
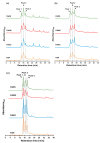
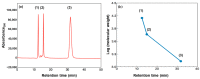
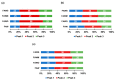
The extraterrestrial delivery of organics to primitive Earth has been supported by many laboratory and space experiments. Minerals played an important role in the evolution of meteoritic organic matter. In this study, we simulated aqueous alteration in small bodies by using a solution mixture of H2CO and NH3 in the presence of water at 150 °C under different heating durations, which produced amino acids after acid hydrolysis. Moreover, minerals were added to the previous mixture to examine their catalyzing/inhibiting impact on amino acid formation. Without minerals, glycine was the dominant amino acid obtained at 1 d of the heating experiment, while alanine and β-alanine increased significantly and became dominant after 3 to 7 d. Minerals enhanced the yield of amino acids at short heating duration (1 d); however, they induced their decomposition at longer heating duration (7 d). Additionally, montmorillonite enhanced amino acid production at 1 d, while olivine and serpentine enhanced production at 3 d. Molecular weight distribution in the whole of the products obtained by gel chromatography showed that minerals enhanced both decomposition and combination of molecules. Our results indicate that minerals affected the formation of amino acids in aqueous environments in small Solar System bodies and that the amino acids could have different response behaviors according to different minerals.
Keywords: amino acids; ammonia; aqueous alteration; formaldehyde; olivine; phyllosilicates; small Solar System bodies.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Gamma-Ray-Induced Amino Acid Formation during Aqueous Alteration in Small Bodies: The Effects of Compositions of Starting Solutions.Life (Basel). 2024 Jan 9;14(1):103. doi: 10.3390/life14010103. Life (Basel). 2024. PMID: 38255718 Free PMC article.
-
Hydrothermal Decomposition of Amino Acids and Origins of Prebiotic Meteoritic Organic Compounds.ACS Earth Space Chem. 2018 Apr 11;2(6):588-598. doi: 10.1021/acsearthspacechem.8b00025. Epub 2018 Jun 21. ACS Earth Space Chem. 2018. PMID: 32637854 Free PMC article.
-
Formation and processing of organics in the early solar system.Space Sci Rev. 1999;90(1-2):275-88. doi: 10.1007/978-94-011-4211-3_25. Space Sci Rev. 1999. PMID: 11543289 Review.
-
One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity.Sci Adv. 2017 Mar 17;3(3):e1602093. doi: 10.1126/sciadv.1602093. eCollection 2017 Mar. Sci Adv. 2017. PMID: 28345041 Free PMC article.
-
d-Amino acids in molecular evolution in space - Absolute asymmetric photolysis and synthesis of amino acids by circularly polarized light.Biochim Biophys Acta Proteins Proteom. 2018 Jul;1866(7):743-758. doi: 10.1016/j.bbapap.2018.01.004. Epub 2018 Jan 31. Biochim Biophys Acta Proteins Proteom. 2018. PMID: 29357311 Review.
Cited by
-
Gamma-Ray-Induced Amino Acid Formation during Aqueous Alteration in Small Bodies: The Effects of Compositions of Starting Solutions.Life (Basel). 2024 Jan 9;14(1):103. doi: 10.3390/life14010103. Life (Basel). 2024. PMID: 38255718 Free PMC article.
-
Gamma-Ray-Induced Amino Acid Formation in Aqueous Small Bodies in the Early Solar System.ACS Cent Sci. 2022 Dec 28;8(12):1664-1671. doi: 10.1021/acscentsci.2c00588. Epub 2022 Dec 7. ACS Cent Sci. 2022. PMID: 36589881 Free PMC article.
-
Experimental and Theoretical Constraints on Amino Acid Formation from PAHs in Asteroidal Settings.ACS Earth Space Chem. 2022 Mar 17;6(3):468-481. doi: 10.1021/acsearthspacechem.1c00329. Epub 2022 Feb 15. ACS Earth Space Chem. 2022. PMID: 35330631 Free PMC article.
References
-
- Robert F., Epstein S. The concentration and isotopic composition of hydrogen, carbon and nitrogen in carbonaceous meteorites. Geochim. Cosmochim. Acta. 1982;46:81–95. doi: 10.1016/0016-7037(82)90293-9. - DOI
-
- Pearson V.K., Sephton M.A., Franchi I.A., Gibson J.M., Gilmour I. Carbon and nitrogen in carbonaceous chondrites: Elemental abundances and stable isotopic compositions. Meteorit. Planet. Sci. 2006;41:1899–1918. doi: 10.1111/j.1945-5100.2006.tb00459.x. - DOI
-
- Alexander C.M.O., Fogel M., Yabuta H., Cody G.D. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim. Cosmochim. Acta. 2007;71:4380–4403. doi: 10.1016/j.gca.2007.06.052. - DOI
-
- Botta O., Bada J.L. Extraterrestrial Organic Compounds in Meteorites. Surv. Geophys. 2002;23:411–467. doi: 10.1023/A:1020139302770. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

