A Nanoparticle-Based Trivalent Vaccine Targeting the Glycan Binding VP8* Domains of Rotaviruses
- PMID: 33419150
- PMCID: PMC7825513
- DOI: 10.3390/v13010072
A Nanoparticle-Based Trivalent Vaccine Targeting the Glycan Binding VP8* Domains of Rotaviruses
Abstract
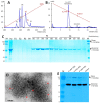
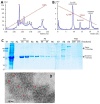
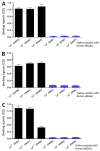
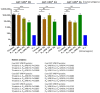
Rotavirus causes severe gastroenteritis in children. Although vaccines are implemented, rotavirus-related diarrhea still claims ~200,000 lives annually worldwide, mainly in low-income settings, pointing to a need for improved vaccine tactics. To meet such a public health need, a P24-VP8* nanoparticle displaying the glycan-binding VP8* domains, the major neutralizing antigens of rotavirus, was generated as a new type of rotavirus vaccine. We reported here our development of a P24-VP8* nanoparticle-based trivalent vaccine. First, we established a method to produce tag-free P24-VP8* nanoparticles presenting the VP8*s of P[8], P[4], and P[6] rotaviruses, respectively, which are the three predominantly circulating rotavirus P types globally. This approach consists of a chemical-based protein precipitation and an ion exchange purification, which may be scaled up for large vaccine production. All three P24-VP8* nanoparticle types self-assembled efficiently with authentic VP8*-glycan receptor binding function. After they were mixed as a trivalent vaccine, we showed that intramuscular immunization of the vaccine elicited high IgG titers specific to the three homologous VP8* types in mice. The resulted mouse sera strongly neutralized replication of all three rotavirus P types in cell culture. Thus, the trivalent P24-VP8* nanoparticles are a promising vaccine candidate for parenteral use against multiple P types of predominant rotaviruses.
Keywords: P24-VP8* nanoparticle; non-replicating rotavirus vaccine; norovirus P domain; rotavirus; rotavirus VP8*; rotavirus vaccine.
Conflict of interest statement
The authors declare no conflict of interest. The funders of this research had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Immune response and protective efficacy of the S particle presented rotavirus VP8* vaccine in mice.Vaccine. 2019 Jul 9;37(30):4103-4110. doi: 10.1016/j.vaccine.2019.05.075. Epub 2019 Jun 11. Vaccine. 2019. PMID: 31201052 Free PMC article.
-
A Pseudovirus Nanoparticle-Based Trivalent Rotavirus Vaccine Candidate Elicits High and Cross P Type Immune Response.Pharmaceutics. 2022 Jul 30;14(8):1597. doi: 10.3390/pharmaceutics14081597. Pharmaceutics. 2022. PMID: 36015223 Free PMC article.
-
Characterization and protective efficacy in an animal model of a novel truncated rotavirus VP8 subunit parenteral vaccine candidate.Vaccine. 2015 May 21;33(22):2606-13. doi: 10.1016/j.vaccine.2015.03.068. Epub 2015 Apr 14. Vaccine. 2015. PMID: 25882173
-
The role and implication of rotavirus VP8∗ in viral infection and vaccine development.Virology. 2025 Aug;609:110563. doi: 10.1016/j.virol.2025.110563. Epub 2025 May 8. Virology. 2025. PMID: 40378555 Review.
-
Genetic diversity of G1P[8] rotavirus VP7 and VP8* antigens in Finland over a 20-year period: No evidence for selection pressure by universal mass vaccination with RotaTeq® vaccine.Infect Genet Evol. 2013 Oct;19:51-8. doi: 10.1016/j.meegid.2013.06.026. Epub 2013 Jul 4. Infect Genet Evol. 2013. PMID: 23831933 Review.
Cited by
-
Bioengineered pseudovirus nanoparticles displaying the HA1 antigens of influenza viruses for enhanced immunogenicity.Nano Res. 2022;15(5):4181-4190. doi: 10.1007/s12274-021-4011-x. Epub 2022 Jan 28. Nano Res. 2022. PMID: 35106126 Free PMC article.
-
The αTSR Domain of Plasmodium Circumsporozoite Protein Bound Heparan Sulfates and Elicited High Titers of Sporozoite Binding Antibody After Displayed by Nanoparticles.Int J Nanomedicine. 2023 Jun 8;18:3087-3107. doi: 10.2147/IJN.S406314. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37312932 Free PMC article.
-
Pseudovirus nanoparticles targeting the receptor binding HA1 domains of influenza viruses elicited high HA1-specific antibody responses and protected mice against mortality caused by influenza virus challenges.Vaccine. 2025 Feb 6;46:126585. doi: 10.1016/j.vaccine.2024.126585. Epub 2024 Dec 7. Vaccine. 2025. PMID: 39648102
-
Insights into recent advancements in human and animal rotavirus vaccines: Exploring new frontiers.Virol Sin. 2025 Feb;40(1):1-14. doi: 10.1016/j.virs.2024.12.001. Epub 2024 Dec 11. Virol Sin. 2025. PMID: 39672271 Free PMC article. Review.
-
A Pseudovirus Nanoparticle Displaying the Vaccinia Virus L1 Protein Elicited High Neutralizing Antibody Titers and Provided Complete Protection to Mice against Mortality Caused by a Vaccinia Virus Challenge.Vaccines (Basel). 2024 Jul 26;12(8):846. doi: 10.3390/vaccines12080846. Vaccines (Basel). 2024. PMID: 39203972 Free PMC article.
References
-
- Vesikari T., Karvonen A., Prymula R., Schuster V., Tejedor J.C., Cohen R., Meurice F., Han H.H., Damaso S., Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: Randomised, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. - DOI - PubMed
-
- Armah G.E., Sow S.O., Breiman R.F., Dallas M.J., Tapia M.D., Feikin D.R., Binka F.N., Steele A.D., Laserson K.F., Ansah N.A., et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: A randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. - DOI - PubMed
-
- Tate J.E., Burton A.H., Boschi-Pinto C., Steele A.D., Duque J., Parashar U.D. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

