Pravastatin Promotes Endothelial Colony-Forming Cell Function, Angiogenic Signaling and Protein Expression In Vitro
- PMID: 33419165
- PMCID: PMC7825508
- DOI: 10.3390/jcm10020183
Pravastatin Promotes Endothelial Colony-Forming Cell Function, Angiogenic Signaling and Protein Expression In Vitro
Abstract
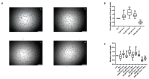
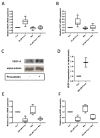
Endothelial dysfunction is a primary feature of several cardiovascular diseases. Endothelial colony-forming cells (ECFCs) represent a highly proliferative subtype of endothelial progenitor cells (EPCs), which are involved in neovascularization and vascular repair. Statins are known to improve the outcome of cardiovascular diseases via pleiotropic effects. We hypothesized that treatment with the 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitor pravastatin increases ECFCs' functional capacities and regulates the expression of proteins which modulate endothelial health in a favourable manner. Umbilical cord blood derived ECFCs were incubated with different concentrations of pravastatin with or without mevalonate, a key intermediate in cholesterol synthesis. Functional capacities such as migration, proliferation and tube formation were addressed in corresponding in vitro assays. mRNA and protein levels or phosphorylation of protein kinase B (AKT), endothelial nitric oxide synthase (eNOS), heme oxygenase-1 (HO-1), vascular endothelial growth factor A (VEGF-A), placental growth factor (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1) and endoglin (Eng) were analyzed by real time PCR or immunoblot, respectively. Proliferation, migration and tube formation of ECFCs were enhanced after pravastatin treatment, and AKT- and eNOS-phosphorylation were augmented. Further, expression levels of HO-1, VEGF-A and PlGF were increased, whereas expression levels of sFlt-1 and Eng were decreased. Pravastatin induced effects were reversible by the addition of mevalonate. Pravastatin induces beneficial effects on ECFC function, angiogenic signaling and protein expression. These effects may contribute to understand the pleiotropic function of statins as well as to provide a promising option to improve ECFCs' condition in cell therapy in order to ameliorate endothelial dysfunction.
Keywords: angiogenesis; cardiovascular disease; cell therapy; endothelial colony-forming cells; endothelial dysfunction; endothelial progenitor cells; pravastatin; preeclampsia; statins; vascular repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Effects of simvastatin, rosuvastatin and pravastatin on soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sENG) secretion from human umbilical vein endothelial cells, primary trophoblast cells and placenta.BMC Pregnancy Childbirth. 2016 May 20;16:117. doi: 10.1186/s12884-016-0902-3. BMC Pregnancy Childbirth. 2016. PMID: 27207105 Free PMC article.
-
Effects of pravastatin on angiogenic and placental hypoxic imbalance in a mouse model of preeclampsia.Reprod Sci. 2014 Jan;21(1):138-45. doi: 10.1177/1933719113492207. Epub 2013 Jun 7. Reprod Sci. 2014. PMID: 23749761
-
Cord blood-derived endothelial colony-forming cell function is disrupted in congenital diaphragmatic hernia.Am J Physiol Lung Cell Mol Physiol. 2016 Jun 1;310(11):L1143-54. doi: 10.1152/ajplung.00357.2015. Epub 2016 Apr 29. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27130531
-
Calcium Signaling in Endothelial Colony Forming Cells in Health and Disease.Adv Exp Med Biol. 2020;1131:1013-1030. doi: 10.1007/978-3-030-12457-1_40. Adv Exp Med Biol. 2020. PMID: 31646543 Review.
-
Systemic lupus erythematosus, endothelial progenitor cells and intracellular Ca2+ signaling: A novel approach for an old disease.J Autoimmun. 2020 Aug;112:102486. doi: 10.1016/j.jaut.2020.102486. Epub 2020 May 29. J Autoimmun. 2020. PMID: 32482487 Review.
Cited by
-
Role of endothelial colony forming cells (ECFCs) Tetrahydrobiopterin (BH4) in determining ECFCs functionality in coronary artery disease (CAD) patients.Sci Rep. 2022 Feb 23;12(1):3076. doi: 10.1038/s41598-022-06758-8. Sci Rep. 2022. PMID: 35197509 Free PMC article.
-
Paradoxical effects of statins on endothelial and cancer cells: the impact of concentrations.Cancer Cell Int. 2023 Mar 10;23(1):43. doi: 10.1186/s12935-023-02890-1. Cancer Cell Int. 2023. PMID: 36899388 Free PMC article. Review.
-
The Effects of Prenatal Pravastatin Treatment in the Rabbit Fetal Growth Restriction Model.Biomedicines. 2023 Sep 30;11(10):2685. doi: 10.3390/biomedicines11102685. Biomedicines. 2023. PMID: 37893059 Free PMC article.
-
Cyclosporine A and Tacrolimus Induce Functional Impairment and Inflammatory Reactions in Endothelial Progenitor Cells.Int J Mol Sci. 2021 Sep 8;22(18):9696. doi: 10.3390/ijms22189696. Int J Mol Sci. 2021. PMID: 34575860 Free PMC article.
-
Mechanisms by which statins protect endothelial cells from radiation-induced injury in the carotid artery.Front Cardiovasc Med. 2023 Jun 19;10:1133315. doi: 10.3389/fcvm.2023.1133315. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37404737 Free PMC article.
References
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

