N-Glycosylation as a Tool to Study Antithrombin Secretion, Conformation, and Function
- PMID: 33419227
- PMCID: PMC7825591
- DOI: 10.3390/ijms22020516
N-Glycosylation as a Tool to Study Antithrombin Secretion, Conformation, and Function
Abstract
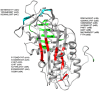
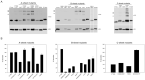
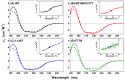
N-linked glycosylation is a crucial post-translational modification involved in protein folding, function, and clearance. N-linked glycosylation is also used therapeutically to enhance the half-lives of many proteins. Antithrombin, a serpin with four potential N-glycosylation sites, plays a pivotal role in hemostasis, wherein its deficiency significantly increases thrombotic risk. In this study, we used the introduction of N-glycosylation sites as a tool to explore what effect this glycosylation has on the protein folding, secretion, and function of this key anticoagulant. To accomplish this task, we introduced an additional N-glycosylation sequence in each strand. Interestingly, all regions that likely fold rapidly or were surrounded by lysines were not glycosylated even though an N-glycosylation sequon was present. The new sequon in the strands of the A- and B-sheets reduced secretion, and the B-sheet was more sensitive to these changes. However, the mutations in the strands of the C-sheet allowed correct folding and secretion, which resulted in functional variants. Therefore, our study revealed crucial regions for antithrombin secretion and could potentially apply to all serpins. These results could also help us understand the functional effects of natural variants causing type-I deficiencies.
Keywords: Glycosylation; antithrombin; bioengineering; coagulation; folding; serpin; thrombosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lijfering W.M., Brouwer J.L.P., Veeger N.J.G.M., Bank I., Coppens M., Middeldorp S., Hamulyák K., Prins M.H., Büller H.R., Van Der Meer J. Selective testing for thrombophilia in patients with first venous thrombosis: Results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood. 2009;113:5314–5322. doi: 10.1182/blood-2008-10-184879. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

