Axitinib in Ponatinib-Resistant B-Cell Acute Lymphoblastic Leukemia Harboring a T315L Mutation
- PMID: 33419251
- PMCID: PMC7765866
- DOI: 10.3390/ijms21249724
Axitinib in Ponatinib-Resistant B-Cell Acute Lymphoblastic Leukemia Harboring a T315L Mutation
Abstract
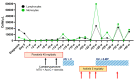
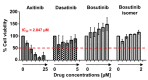
Adult acute lymphoblastic leukemia (ALL) with BCR-ABL1 rearrangement (Philadelphia chromosome, Ph) is a hematological aggressive disease with a fatal outcome in more than 50% of cases. Tyrosine kinase inhibitors (TKIs) targeting the activity of BCR-ABL1 protein have improved the prognosis; however, relapses are frequent because of acquired somatic mutations in the BCR-ABL1 kinase domain causing resistance to first, second and third generation TKIs. Axitinib has shown in vitro and ex vivo activity in blocking ABL1; however, clinical trials exploring its efficacy in ALL are missing. Here, we presented a 77-year-old male with a diagnosis of Ph positive ALL resistant to ponatinib and carrying a rare threonine to leucine (T315L) mutation on BCR-ABL1 gene. The patient was treated with axitinib at 5 mg/twice daily as salvage therapy showing an immediate although transient benefit with an overall survival of 9.3 months. Further dose-finding and randomized clinical trials are required to assess the real efficacy of axitinib for adult Ph positive ALL resistant to third generation TKIs.
Keywords: TKI inhibitor; acute lymphoblastic leukemia; axitinib; ponatinib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Ponatinib in the treatment of chronic myeloid leukemia and philadelphia chromosome positive acute lymphoblastic leukemia.Future Oncol. 2019 Jan;15(3):257-269. doi: 10.2217/fon-2018-0371. Epub 2018 Sep 25. Future Oncol. 2019. PMID: 30251548 Review.
-
Efficacy and safety of ponatinib for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a case series from a single institute.Int J Hematol. 2021 Aug;114(2):199-204. doi: 10.1007/s12185-021-03156-0. Epub 2021 Apr 27. Int J Hematol. 2021. PMID: 33907977
-
Ponatinib in the Treatment of Chronic Myeloid Leukemia and Philadelphia Chromosome-Positive Acute Leukemia: Recommendations of a German Expert Consensus Panel with Focus on Cardiovascular Management.Acta Haematol. 2020;143(3):217-231. doi: 10.1159/000501927. Epub 2019 Oct 7. Acta Haematol. 2020. PMID: 31590170 Free PMC article. Review.
-
Ponatinib after failure of second-generation tyrosine kinase inhibitor in resistant chronic-phase chronic myeloid leukemia.Am J Hematol. 2022 Nov;97(11):1419-1426. doi: 10.1002/ajh.26686. Epub 2022 Aug 30. Am J Hematol. 2022. PMID: 36054756 Free PMC article.
-
Ponatinib in refractory Philadelphia chromosome-positive leukemias.N Engl J Med. 2012 Nov 29;367(22):2075-88. doi: 10.1056/NEJMoa1205127. N Engl J Med. 2012. PMID: 23190221 Free PMC article. Clinical Trial.
Cited by
-
Kaposi's sarcoma associated with chronic myeloid leukemia and imatinib mesylate therapy.Clin Case Rep. 2022 Jun 5;10(6):e05919. doi: 10.1002/ccr3.5919. eCollection 2022 Jun. Clin Case Rep. 2022. PMID: 35677856 Free PMC article.
-
Case report: BCR-ABL-positive acute lymphoblastic leukemia with bone destruction: a treatment dilemma.Front Oncol. 2024 Feb 21;14:1356311. doi: 10.3389/fonc.2024.1356311. eCollection 2024. Front Oncol. 2024. PMID: 38450181 Free PMC article.
References
-
- Mullighan C.G., Collins-Underwood J.R., Phillips L.A., Loudin M.G., Liu W., Zhang J., Ma J., Coustan-Smith E., Harvey R.C., Willman C.L., et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat. Genet. 2009;41:1243–1246. doi: 10.1038/ng.469. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

