Light Stability, Pro-Apoptotic and Genotoxic Properties of Silver (I) Complexes of Metronidazole and 4-Hydroxymethylpyridine against Pancreatic Cancer Cells In Vitro
- PMID: 33419296
- PMCID: PMC7767315
- DOI: 10.3390/cancers12123848
Light Stability, Pro-Apoptotic and Genotoxic Properties of Silver (I) Complexes of Metronidazole and 4-Hydroxymethylpyridine against Pancreatic Cancer Cells In Vitro
Abstract
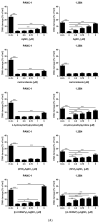
Antimicrobial properties of silver (I) ion and its complexes are well recognized. However, recent studies suggest that both silver (I) ion and its complexes possess anticancer activity associated with oxidative stress-induced apoptosis of various cancer cells. In this study, we aimed to investigate whether silver nitrate and its complexes with metronidazole and 4-hydroxymethylpyridine exert anticancer action against human pancreatic cancer cell lines (PANC-1 and 1.2B4). In the study, we compared decomposition speed for silver complexes under the influence of daylight and UV-A (ultraviolet-A) rays. We employed the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazonium bromide) assay to evaluate the cytotoxicity and the alkaline comet assay to determine genotoxicity of silver nitrate and its complexes. Flow cytometry and the Annexin V-FITC/PI apoptosis detection kit were used to detect the apoptosis of human pancreatic cancer cells. We found a dose dependent decrease of both pancreatic cancer cell line viability after exposure to silver nitrate and its complexes. The flow cytometry analysis confirmed that cell death occurred mainly via apoptosis. We also documented that the studied compounds induced DNA damage. Metronidazole and 4-hydroxymethylpyridine alone did not significantly affect viability and level of DNA damage of pancreatic cancer cell lines. Complex compounds showed better stability than AgNO3, which decomposed slower than when exposed to light. UV-A significantly influences the speed of silver salt decomposition reaction. To conclude, obtained data demonstrated that silver nitrate and its complexes exerted anticancer action against human pancreatic cancer cells.
Keywords: 4-hydroxymethylpyridine; UV-A rays; apoptosis; complex; cytotoxicity; genotoxicity; light stability; medicinal chemistry; metronidazole; pancreatic cancer cells; silver (I).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
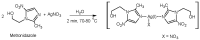
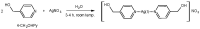





Similar articles
-
Multifunctional Silver(I) Complexes with Metronidazole Drug Reveal Antimicrobial Properties and Antitumor Activity against Human Hepatoma and Colorectal Adenocarcinoma Cells.Cancers (Basel). 2022 Feb 11;14(4):900. doi: 10.3390/cancers14040900. Cancers (Basel). 2022. PMID: 35205647 Free PMC article.
-
Silver(I) Complexes of the Pharmaceutical Agents Metronidazole and 4-Hydroxymethylpyridine: Comparison of Cytotoxic Profile for Potential Clinical Application.Molecules. 2019 May 21;24(10):1949. doi: 10.3390/molecules24101949. Molecules. 2019. PMID: 31117201 Free PMC article.
-
Genotoxicity and apoptotic effect of silver(I) complexes with mixed-ligands of thiosemicarbazones and diphenyl(p-tolyl)phosphine on malignant melanoma cells, SK-MEL-28.Mutat Res Genet Toxicol Environ Mutagen. 2023 Feb-Mar;886:503581. doi: 10.1016/j.mrgentox.2022.503581. Epub 2022 Dec 23. Mutat Res Genet Toxicol Environ Mutagen. 2023. PMID: 36868695
-
Cytotoxic, anti-proliferative and apoptotic effects of silver nitrate against H-ras transformed 5RP7.Cytotechnology. 2016 Oct;68(5):1727-35. doi: 10.1007/s10616-015-9922-5. Epub 2015 Oct 26. Cytotechnology. 2016. PMID: 26499861 Free PMC article.
-
Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway.Phytomedicine. 2018 Jan 15;39:10-16. doi: 10.1016/j.phymed.2017.12.001. Epub 2017 Dec 5. Phytomedicine. 2018. PMID: 29433670
Cited by
-
Multifunctional Silver(I) Complexes with Metronidazole Drug Reveal Antimicrobial Properties and Antitumor Activity against Human Hepatoma and Colorectal Adenocarcinoma Cells.Cancers (Basel). 2022 Feb 11;14(4):900. doi: 10.3390/cancers14040900. Cancers (Basel). 2022. PMID: 35205647 Free PMC article.
-
Synergistic Potential of Antibiotics with Cancer Treatments.Cancers (Basel). 2024 Dec 28;17(1):59. doi: 10.3390/cancers17010059. Cancers (Basel). 2024. PMID: 39796688 Free PMC article. Review.
-
Silver, Its Salts and Application in Medicine and Pharmacy.Int J Mol Sci. 2023 Oct 29;24(21):15723. doi: 10.3390/ijms242115723. Int J Mol Sci. 2023. PMID: 37958707 Free PMC article. Review.
-
Silver(I) Complexes Based on Oxadiazole-Functionalized α-Aminophosphonate: Synthesis, Structural Study, and Biological Activities.Molecules. 2022 Nov 22;27(23):8131. doi: 10.3390/molecules27238131. Molecules. 2022. PMID: 36500224 Free PMC article.
-
Silver Complexes of Miconazole and Metronidazole: Potential Candidates for Melanoma Treatment.Int J Mol Sci. 2024 May 7;25(10):5081. doi: 10.3390/ijms25105081. Int J Mol Sci. 2024. PMID: 38791121 Free PMC article.
References
-
- Kontek R., Matlawska-Wasowska K., Kalinowska-Lis U., Kontek B., Ochocki J. Evaluation of cytotoxity of new trans-palladium(II) complex in human cells in vitro. Acta Pol. Pharm. 2011;68:127–136. - PubMed
-
- Kasprzak M., Fabijanska M., Checinska L., Studzian K., Markowicz-Piasecka M., Sikora J., Mikiciuk-Olasik E., Ochocki J. Small differences in structure, large difference in activity-Comparison of the new Ru (II) -3-hydroxyiminoflavanone complex with analogous Ru (II) compounds. Inorg. Chim. Acta. 2017;457:69–80. doi: 10.1016/j.ica.2016.11.021. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

