Implication of the Association of Fibrinogen Citrullination and Osteoclastogenesis in Bone Destruction in Rheumatoid Arthritis
- PMID: 33419308
- PMCID: PMC7766778
- DOI: 10.3390/cells9122720
Implication of the Association of Fibrinogen Citrullination and Osteoclastogenesis in Bone Destruction in Rheumatoid Arthritis
Abstract
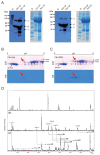
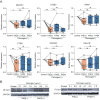
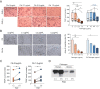
Immune complexes containing citrullinated fibrinogen are present in the sera and synovium of rheumatoid arthritis patients and potentially contribute to synovitis. However, fibrinogen can inhibit the osteoclastogenesis of precursor cells. We investigated the direct effect of citrullinated fibrinogen on osteoclastogenesis to understand the role of citrullination on bone erosion of rheumatoid arthritis patients. We evaluated the fibrinogen citrullination sites using mass spectrometry and quantified osteoclast-related protein and gene expression levels by Western blotting, microarray, and real-time polymerase chain reaction. Differences in spectral peaks were noted between fibrinogen and citrullinated fibrinogen at five sites in α-chains, two sites in β-chains, and one site in a γ-chain. Transcriptome changes induced by fibrinogen and citrullinated fibrinogen were identified and differentially expressed genes grouped into three distinctive modules. Fibrinogen was then citrullinated in vitro using peptidylarginine deiminase. When increasing doses of soluble fibrinogen and citrullinated fibrinogen were applied to human CD14+ monocytes, citrullination restored osteoclastogenesis-associated changes, including NF-ATc1 and ß3-integrin. Finally, citrullination rescued the number of osteoclasts by restoring fibrinogen-induced suppression of osteoclastogenesis. Taken together, the results indicate that the inhibitory function of fibrinogen on osteoclastogenesis is reversed by citrullination and suggest that citrullinated fibrinogen may contribute to erosive bone destruction in rheumatoid arthritis.
Keywords: citrullinated fibrinogen; osteoclastogenesis; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

