Advancements in the Blood-Brain Barrier Penetrating Nanoplatforms for Brain Related Disease Diagnostics and Therapeutic Applications
- PMID: 33419339
- PMCID: PMC7766280
- DOI: 10.3390/polym12123055
Advancements in the Blood-Brain Barrier Penetrating Nanoplatforms for Brain Related Disease Diagnostics and Therapeutic Applications
Abstract
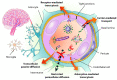
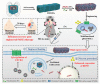
Noninvasive treatments to treat the brain-related disorders have been paying more significant attention and it is an emerging topic. However, overcoming the blood brain barrier (BBB) is a key obstacle to most of the therapeutic drugs to enter into the brain tissue, which significantly results in lower accumulation of therapeutic drugs in the brain. Thus, administering the large quantity/doses of drugs raises more concerns of adverse side effects. Nanoparticle (NP)-mediated drug delivery systems are seen as potential means of enhancing drug transport across the BBB and to targeted brain tissue. These systems offer more accumulation of therapeutic drugs at the tumor site and prolong circulation time in the blood. In this review, we summarize the current knowledge and advancements on various nanoplatforms (NF) and discusses the use of nanoparticles for successful cross of BBB to treat the brain-related disorders such as brain tumors, Alzheimer's disease, Parkinson's disease, and stroke.
Keywords: Alzheimer’s disease; Parkinson’s disease; blood brain barrier; brain tumor; nanoparticle; stroke.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Therapeutic nanoplatforms and delivery strategies for neurological disorders.Nano Converg. 2018 Nov 30;5(1):35. doi: 10.1186/s40580-018-0168-8. Nano Converg. 2018. PMID: 30499047 Free PMC article. Review.
-
Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases.J Control Release. 2016 Aug 10;235:34-47. doi: 10.1016/j.jconrel.2016.05.044. Epub 2016 May 18. J Control Release. 2016. PMID: 27208862 Review.
-
The Therapeutic Benefits of Intravenously Administrated Nanoparticles in Stroke and Age-related Neurodegenerative Diseases.Curr Pharm Des. 2022;28(24):1985-2000. doi: 10.2174/1381612828666220608093639. Curr Pharm Des. 2022. PMID: 35676838
-
Treating Alzheimer's disease using nanoparticle-mediated drug delivery strategies/systems.Ageing Res Rev. 2024 Jun;97:102291. doi: 10.1016/j.arr.2024.102291. Epub 2024 Apr 12. Ageing Res Rev. 2024. PMID: 38614367 Review.
-
Current Strategies to Enhance Delivery of Drugs across the Blood-Brain Barrier.Pharmaceutics. 2022 May 4;14(5):987. doi: 10.3390/pharmaceutics14050987. Pharmaceutics. 2022. PMID: 35631573 Free PMC article. Review.
Cited by
-
The Major Hypotheses of Alzheimer's Disease: Related Nanotechnology-Based Approaches for Its Diagnosis and Treatment.Cells. 2023 Nov 21;12(23):2669. doi: 10.3390/cells12232669. Cells. 2023. PMID: 38067098 Free PMC article. Review.
-
Transportation of Single-Domain Antibodies through the Blood-Brain Barrier.Biomolecules. 2021 Jul 31;11(8):1131. doi: 10.3390/biom11081131. Biomolecules. 2021. PMID: 34439797 Free PMC article. Review.
-
Dendrimers in Alzheimer's Disease: Recent Approaches in Multi-Targeting Strategies.Pharmaceutics. 2023 Mar 10;15(3):898. doi: 10.3390/pharmaceutics15030898. Pharmaceutics. 2023. PMID: 36986759 Free PMC article. Review.
-
Synergistic activity of nootropic herbs as potent therapeutics for Alzheimer's disease: A cheminformatics, pharmacokinetics, and system pharmacology approach.J Alzheimers Dis Rep. 2024 Dec 23;8(1):1745-1762. doi: 10.1177/25424823241307019. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 40034353 Free PMC article.
-
Mimosine functionalized gold nanoparticles (Mimo-AuNPs) suppress β-amyloid aggregation and neuronal toxicity.Bioact Mater. 2021 May 10;6(12):4491-4505. doi: 10.1016/j.bioactmat.2021.04.029. eCollection 2021 Dec. Bioact Mater. 2021. PMID: 34027236 Free PMC article.
References
-
- Bors L.A., Erdő F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019;87:6. doi: 10.3390/scipharm87010006. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

