Persistence of intramyocardially transplanted murine induced pluripotent stem cell-derived cardiomyocytes from different developmental stages
- PMID: 33419458
- PMCID: PMC7792075
- DOI: 10.1186/s13287-020-02089-5
Persistence of intramyocardially transplanted murine induced pluripotent stem cell-derived cardiomyocytes from different developmental stages
Abstract
Background: Induced pluripotent stem cell-derived cardiomyocytes (iPSC-CM) are regarded as promising cell type for cardiac cell replacement therapy, but it is not known whether the developmental stage influences their persistence and functional integration in the host tissue, which are crucial for a long-term therapeutic benefit. To investigate this, we first tested the cell adhesion capability of murine iPSC-CM in vitro at three different time points during the differentiation process and then examined cell persistence and quality of electrical integration in the infarcted myocardium in vivo.
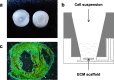
Methods: To test cell adhesion capabilities in vitro, iPSC-CM were seeded on fibronectin-coated cell culture dishes and decellularized ventricular extracellular matrix (ECM) scaffolds. After fixed periods of time, stably attached cells were quantified. For in vivo experiments, murine iPSC-CM expressing enhanced green fluorescent protein was injected into infarcted hearts of adult mice. After 6-7 days, viable ventricular tissue slices were prepared to enable action potential (AP) recordings in transplanted iPSC-CM and surrounding host cardiomyocytes. Afterwards, slices were lysed, and genomic DNA was prepared, which was then used for quantitative real-time PCR to evaluate grafted iPSC-CM count.
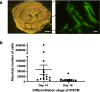
Results: The in vitro results indicated differences in cell adhesion capabilities between day 14, day 16, and day 18 iPSC-CM with day 14 iPSC-CM showing the largest number of attached cells on ECM scaffolds. After intramyocardial injection, day 14 iPSC-CM showed a significant higher cell count compared to day 16 iPSC-CM. AP measurements revealed no significant difference in the quality of electrical integration and only minor differences in AP properties between d14 and d16 iPSC-CM.
Conclusion: The results of the present study demonstrate that the developmental stage at the time of transplantation is crucial for the persistence of transplanted iPSC-CM. iPSC-CM at day 14 of differentiation showed the highest persistence after transplantation in vivo, which may be explained by a higher capability to adhere to the extracellular matrix.
Keywords: Cell persistence; Cell therapy; Induced pluripotent stem cell-derived cardiomyocytes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Transplantation of human induced pluripotent stem cell-derived cardiomyocytes improves myocardial function and reverses ventricular remodeling in infarcted rat hearts.Stem Cell Res Ther. 2020 Feb 21;11(1):73. doi: 10.1186/s13287-020-01602-0. Stem Cell Res Ther. 2020. PMID: 32085809 Free PMC article.
-
Bioluminescent imaging of genetically selected induced pluripotent stem cell-derived cardiomyocytes after transplantation into infarcted heart of syngeneic recipients.PLoS One. 2014 Sep 16;9(9):e107363. doi: 10.1371/journal.pone.0107363. eCollection 2014. PLoS One. 2014. PMID: 25226590 Free PMC article.
-
Functional and Electrical Integration of Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Myocardial Infarction Rat Heart.Cell Transplant. 2015;24(12):2479-89. doi: 10.3727/096368914X685799. Epub 2015 Jan 20. Cell Transplant. 2015. PMID: 25606821
-
Spaceflight Promoted Myocardial Differentiation of Induced Pluripotent Stem Cells: Results from Tianzhou-1 Space Mission.Stem Cells Dev. 2019 Mar 15;28(6):357-360. doi: 10.1089/scd.2018.0240. Epub 2019 Feb 25. Stem Cells Dev. 2019. PMID: 30654722 Review.
-
The Structural and the Functional Aspects of Intercellular Communication in iPSC-Cardiomyocytes.Int J Mol Sci. 2022 Apr 18;23(8):4460. doi: 10.3390/ijms23084460. Int J Mol Sci. 2022. PMID: 35457277 Free PMC article. Review.
Cited by
-
From genome editing to blastocyst complementation: A new horizon in heart transplantation?JTCVS Tech. 2022 Jan 21;12:177-184. doi: 10.1016/j.xjtc.2022.01.012. eCollection 2022 Apr. JTCVS Tech. 2022. PMID: 35403039 Free PMC article. No abstract available.
-
CRISPR-Cas9 immune-evasive hESCs are rejected following transplantation into immunocompetent mice.Front Genome Ed. 2024 May 28;6:1403395. doi: 10.3389/fgeed.2024.1403395. eCollection 2024. Front Genome Ed. 2024. PMID: 38863835 Free PMC article.
-
Maturation of pluripotent stem cell-derived cardiomyocytes: limitations and challenges from metabolic aspects.Stem Cell Res Ther. 2024 Oct 8;15(1):354. doi: 10.1186/s13287-024-03961-4. Stem Cell Res Ther. 2024. PMID: 39380099 Free PMC article. Review.
-
Living myocardial slices: Advancing arrhythmia research.Front Physiol. 2023 Jan 13;14:1076261. doi: 10.3389/fphys.2023.1076261. eCollection 2023. Front Physiol. 2023. PMID: 36711023 Free PMC article. Review.
-
Cardiac regeneration: Options for repairing the injured heart.Front Cardiovasc Med. 2023 Jan 12;9:981982. doi: 10.3389/fcvm.2022.981982. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36712238 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

