Exposure to diesel exhaust particles results in altered lung microbial profiles, associated with increased reactive oxygen species/reactive nitrogen species and inflammation, in C57Bl/6 wildtype mice on a high-fat diet
- PMID: 33419468
- PMCID: PMC7796587
- DOI: 10.1186/s12989-020-00393-9
Exposure to diesel exhaust particles results in altered lung microbial profiles, associated with increased reactive oxygen species/reactive nitrogen species and inflammation, in C57Bl/6 wildtype mice on a high-fat diet
Abstract
Background: Exposure to traffic-generated emissions is associated with the development and exacerbation of inflammatory lung disorders such as chronic obstructive pulmonary disorder (COPD) and idiopathic pulmonary fibrosis (IPF). Although many lung diseases show an expansion of Proteobacteria, the role of traffic-generated particulate matter pollutants on the lung microbiota has not been well-characterized. Thus, we investigated the hypothesis that exposure to diesel exhaust particles (DEP) can alter commensal lung microbiota, thereby promoting alterations in the lung's immune and inflammatory responses. We aimed to understand whether diet might also contribute to the alteration of the commensal lung microbiome, either alone or related to exposure. To do this, we used male C57Bl/6 mice (4-6-week-old) on either regular chow (LF) or high-fat (HF) diet (45% kcal fat), randomly assigned to be exposed via oropharyngeal aspiration to 35 μg DEP, suspended in 35 μl 0.9% sterile saline or sterile saline only (control) twice a week for 30 days. A separate group of study animals on the HF diet was concurrently treated with 0.3 g/day of Winclove Ecologic® Barrier probiotics in their drinking water throughout the study.
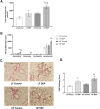
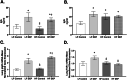
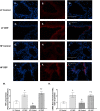
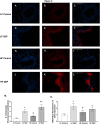
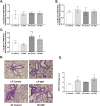
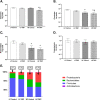
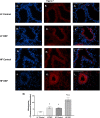
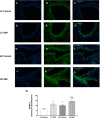
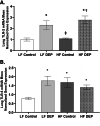
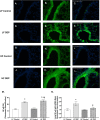
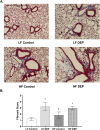
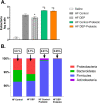
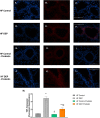
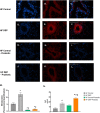
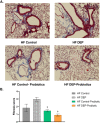
Results: Our results show that DEP-exposure increases lung tumor necrosis factor (TNF)-α, interleukin (IL)-10, Toll-like receptor (TLR)-2, TLR-4, and the nuclear factor kappa B (NF-κB) histologically and by RT-qPCR, as well as Immunoglobulin A (IgA) and Immunoglobulin G (IgG) in the bronchoalveolar lavage fluid (BALF), as quantified by ELISA. We also observed an increase in macrophage infiltration and peroxynitrite, a marker of reactive oxygen species (ROS) + reactive nitrogen species (RNS), immunofluorescence staining in the lungs of DEP-exposed and HF-diet animals, which was further exacerbated by concurrent DEP-exposure and HF-diet consumption. Histological examinations revealed enhanced inflammation and collagen deposition in the lungs DEP-exposed mice, regardless of diet. We observed an expansion of Proteobacteria, by qPCR of bacterial 16S rRNA, in the BALF of DEP-exposed mice on the HF diet, which was diminished with probiotic-treatment.
Conclusions: Our findings suggest that exposure to DEP causes persistent and sustained inflammation and bacterial alterations in a ROS-RNS mediated fashion, which is exacerbated by concurrent consumption of an HF diet.
Keywords: Diesel particulate matter; Inflammation; Lung microbiome; Probiotics; Reactive nitrogen species; Reactive oxygen species.
Conflict of interest statement
Probiotics were provided by Winclove Probiotics and funding from a grant received from the National Institute of Environmental Health Sciences at National Institute of Health was used to conduct some of the studies described, herein; however, the authors declare no conflict of interest or financial gains to these entities associated with this publication.
Figures
Similar articles
-
Inhaled diesel exhaust particles result in microbiome-related systemic inflammation and altered cardiovascular disease biomarkers in C57Bl/6 male mice.Part Fibre Toxicol. 2022 Feb 9;19(1):10. doi: 10.1186/s12989-022-00452-3. Part Fibre Toxicol. 2022. PMID: 35135577 Free PMC article.
-
Diesel exhaust particle inhalation in conjunction with high-fat diet consumption alters the expression of pulmonary SARS-COV-2 infection pathways, which is mitigated by probiotic treatment in C57BL/6 male mice.Part Fibre Toxicol. 2024 Sep 29;21(1):40. doi: 10.1186/s12989-024-00601-w. Part Fibre Toxicol. 2024. PMID: 39343929 Free PMC article.
-
Probiotics Function as Immunomodulators in the Intestine in C57Bl/6 Male Mice Exposed to Inhaled Diesel Exhaust Particles on a High-Fat Diet.Cells. 2022 Apr 25;11(9):1445. doi: 10.3390/cells11091445. Cells. 2022. PMID: 35563751 Free PMC article.
-
Effect of diesel exhaust particulate (DEP) on immune responses: contributions of particulate versus organic soluble components.J Toxicol Environ Health A. 2004 Feb 13;67(3):221-31. doi: 10.1080/15287390490266891. J Toxicol Environ Health A. 2004. PMID: 14681077 Review.
-
Causation by Diesel Exhaust Particles of Endothelial Dysfunctions in Cytotoxicity, Pro-inflammation, Permeability, and Apoptosis Induced by ROS Generation.Cardiovasc Toxicol. 2017 Oct;17(4):384-392. doi: 10.1007/s12012-016-9364-0. Cardiovasc Toxicol. 2017. PMID: 26965709 Review.
Cited by
-
Exposure to diesel exhaust particulates and desert sand dust generates microvesicle particles and platelet-activating factor agonists.Skin Res Technol. 2023 Apr;29(4):e13312. doi: 10.1111/srt.13312. Skin Res Technol. 2023. PMID: 37113092 Free PMC article. No abstract available.
-
Aldehyde metabolism governs resilience of mucociliary clearance to air pollution exposure.J Clin Invest. 2025 May 15;135(14):e191276. doi: 10.1172/JCI191276. eCollection 2025 Jul 15. J Clin Invest. 2025. PMID: 40408364 Free PMC article.
-
Translocation and Dissemination of Gut Bacteria after Severe Traumatic Brain Injury.Microorganisms. 2022 Oct 21;10(10):2082. doi: 10.3390/microorganisms10102082. Microorganisms. 2022. PMID: 36296362 Free PMC article.
-
The treatment of Qibai Pingfei Capsule on chronic obstructive pulmonary disease may be mediated by Th17/Treg balance and gut-lung axis microbiota.J Transl Med. 2022 Jun 21;20(1):281. doi: 10.1186/s12967-022-03481-w. J Transl Med. 2022. PMID: 35729584 Free PMC article.
-
Involvement of Fgf2-mediated tau protein phosphorylation in cognitive deficits induced by sevoflurane in aged rats.Mol Med. 2024 Mar 16;30(1):39. doi: 10.1186/s10020-024-00784-0. Mol Med. 2024. PMID: 38493090 Free PMC article.
References
-
- Winterbottom CJ, Shah RJ, Patterson KC, Kreider ME, Panettieri RA, Jr, Rivera-Lebron B, Miller WT, Litzky LA, Penning TM, Heinlen K, Jackson T, Localio AR, Christie JD. Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest. 2018;153(5):1221–1228. doi: 10.1016/j.chest.2017.07.034. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

