A platform trial in practice: adding a new experimental research arm to the ongoing confirmatory FLAIR trial in chronic lymphocytic leukaemia
- PMID: 33419469
- PMCID: PMC7792072
- DOI: 10.1186/s13063-020-04971-2
A platform trial in practice: adding a new experimental research arm to the ongoing confirmatory FLAIR trial in chronic lymphocytic leukaemia
Abstract
Background: The FLAIR trial in chronic lymphocytic leukaemia has a randomised, controlled, open-label, confirmatory, platform design. FLAIR was successfully amended to include an emerging promising experimental therapy to expedite its assessment, greatly reducing the time to reach the primary outcome compared to running a separate trial and without compromising the validity of the research or the ability to recruit to the trial and report the outcomes. The methodological and practical issues are presented, describing how they were addressed to ensure the amendment was a success.
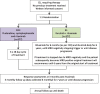
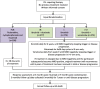
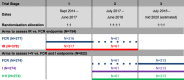
Methods: FLAIR was designed as a two-arm trial requiring 754 patients. In stage 2, two new arms were added: a new experimental arm and a second control arm to protect the trial in case of a change in practice. In stage 3, the original experimental arm was closed as its planned recruitment target was reached. In total, 1516 participants will be randomised to the trial.
Results: The changes to the protocol and randomisation to add and stop arms were made seamlessly without pausing recruitment. The statistical considerations to ensure the results for the original and new hypotheses are unbiased were approved following peer review by oversight committees, Cancer Research UK, ethical and regulatory committees and pharmaceutical partners. These included the use of concurrent comparators in case of any stage effect, appropriate control of the type I error rate and consideration of analysis methods across trial stages. The operational aspects of successfully implementing the amendments are described, including gaining approvals and additional funding, data management requirements and implementation at centres.
Conclusions: FLAIR is an exemplar of how an emerging experimental therapy can be assessed within an existing trial structure without compromising the conduct, reporting or validity of the trial. This strategy offered considerable resource savings and allowed the new experimental therapy to be assessed within a confirmatory trial in the UK years earlier than would have otherwise been possible. Despite the clear efficiencies, treatment arms are rarely added to ongoing trials in practice. This paper demonstrates how this strategy is acceptable, feasible and beneficial to patients and the wider research community.
Trial registration: ISRCTN Registry ISRCTN01844152 . Registered on August 08, 2014.
Keywords: Adding treatment arms; Chronic lymphocytic leukaemia; Complex innovative design; Confirmatory hypotheses; Flexible design; Multi-arm clinical trial; Platform trial; Randomised controlled trial; Statistical methodology; Trial management.
Conflict of interest statement
PH has received research support and honoraria for lecturing from Roche, Janssen, Gilead, Abbvie and Novartis. Since completing this work at the University of Leeds, DRH became an employee of Roche Products Ltd. The other authors declare they have no competing interests.
Figures
References
-
- Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62–70. - PubMed
-
- Berry SM, Connor JT, Lewis RJ. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA. 2015;313(16):1619–1620. - PubMed
-
- Angus DC, Alexander BM, Berry S, Buxton M, Lewis R, Paoloni M, et al. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18(10):797–807. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

