YAP/TAZ inhibition reduces metastatic potential of Ewing sarcoma cells
- PMID: 33419969
- PMCID: PMC7794350
- DOI: 10.1038/s41389-020-00294-8
YAP/TAZ inhibition reduces metastatic potential of Ewing sarcoma cells
Abstract
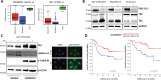
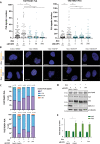
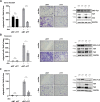
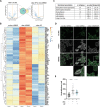
Ewing sarcoma (EwS) is a highly metastatic bone cancer characterized by the ETS fusion oncoprotein EWS-FLI1. EwS cells are phenotypically highly plastic and switch between functionally distinct cell states dependent on EWS-FLI1 fluctuations. Whereas EWS-FLI1high cells proliferate, EWS-FLI1low cells are migratory and invasive. Recently, we reported activation of MRTFB and TEAD, effectors of RhoA and Hippo signalling, upon low EWS-FLI1, orchestrating key steps of the EwS migratory gene expression program. TEAD and its co-activators YAP and TAZ are commonly overexpressed in cancer, providing attractive therapeutic targets. We find TAZ levels to increase in the migratory EWS-FLI1low state and to associate with adverse prognosis in EwS patients. We tested the effects of the potent YAP/TAZ/TEAD complex inhibitor verteporfin on EwS cell migration in vitro and on metastasis in vivo. Verteporfin suppressed expression of EWS-FLI1 regulated cytoskeletal genes involved in actin signalling to the extracellular matrix, effectively blocked F-actin and focal-adhesion assembly and inhibited EwS cell migration at submicromolar concentrations. In a mouse EwS xenograft model, verteporfin treatment reduced relapses at the surgical site and delayed lung metastasis. These data suggest that YAP/TAZ pathway inhibition may prevent EwS cell dissemination and metastasis, justifying further preclinical development of YAP/TAZ inhibitors for EwS treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
EWS-FLI1 perturbs MRTFB/YAP-1/TEAD target gene regulation inhibiting cytoskeletal autoregulatory feedback in Ewing sarcoma.Oncogene. 2017 Oct 26;36(43):5995-6005. doi: 10.1038/onc.2017.202. Epub 2017 Jul 3. Oncogene. 2017. PMID: 28671673 Free PMC article.
-
Hippo pathway effectors YAP1/TAZ induce an EWS-FLI1-opposing gene signature and associate with disease progression in Ewing sarcoma.J Pathol. 2020 Apr;250(4):374-386. doi: 10.1002/path.5379. Epub 2020 Feb 4. J Pathol. 2020. PMID: 31880317
-
The YAP/TAZ Pathway in Osteogenesis and Bone Sarcoma Pathogenesis.Cells. 2020 Apr 15;9(4):972. doi: 10.3390/cells9040972. Cells. 2020. PMID: 32326412 Free PMC article. Review.
-
BET bromodomain inhibitors suppress EWS-FLI1-dependent transcription and the IGF1 autocrine mechanism in Ewing sarcoma.Oncotarget. 2016 Jul 12;7(28):43504-43517. doi: 10.18632/oncotarget.9762. Oncotarget. 2016. PMID: 27259270 Free PMC article.
-
Regulation of Metastasis in Ewing Sarcoma.Cancers (Basel). 2022 Oct 7;14(19):4902. doi: 10.3390/cancers14194902. Cancers (Basel). 2022. PMID: 36230825 Free PMC article. Review.
Cited by
-
Novel sequential therapy with metformin enhances the effects of cisplatin in testicular germ cell tumours via YAP1 signalling.Cancer Cell Int. 2022 Mar 9;22(1):113. doi: 10.1186/s12935-022-02534-w. Cancer Cell Int. 2022. PMID: 35264157 Free PMC article.
-
Inhibition of epithelial cell YAP-TEAD/LOX signaling attenuates pulmonary fibrosis in preclinical models.Nat Commun. 2025 Aug 2;16(1):7099. doi: 10.1038/s41467-025-61795-x. Nat Commun. 2025. PMID: 40753090 Free PMC article.
-
Metabolic landscapes in sarcomas.J Hematol Oncol. 2021 Jul 22;14(1):114. doi: 10.1186/s13045-021-01125-y. J Hematol Oncol. 2021. PMID: 34294128 Free PMC article. Review.
-
Extracellular matrix and Hippo signaling as therapeutic targets of antifibrotic compounds for uterine fibroids.Clin Transl Med. 2021 Jul;11(7):e475. doi: 10.1002/ctm2.475. Clin Transl Med. 2021. PMID: 34323413 Free PMC article. Clinical Trial.
-
EWS/FLI1 Characterization, Activation, Repression, Target Genes and Therapeutic Opportunities in Ewing Sarcoma.Int J Mol Sci. 2023 Oct 14;24(20):15173. doi: 10.3390/ijms242015173. Int J Mol Sci. 2023. PMID: 37894854 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

