An autophagy enhancer ameliorates diabetes of human IAPP-transgenic mice through clearance of amyloidogenic oligomer
- PMID: 33420039
- PMCID: PMC7794419
- DOI: 10.1038/s41467-020-20454-z
An autophagy enhancer ameliorates diabetes of human IAPP-transgenic mice through clearance of amyloidogenic oligomer
Abstract
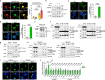
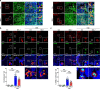
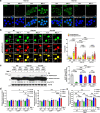
We have reported that autophagy is crucial for clearance of amyloidogenic human IAPP (hIAPP) oligomer, suggesting that an autophagy enhancer could be a therapeutic modality against human diabetes with amyloid accumulation. Here, we show that a recently identified autophagy enhancer (MSL-7) reduces hIAPP oligomer accumulation in human induced pluripotent stem cell-derived β-cells (hiPSC-β-cells) and diminishes oligomer-mediated apoptosis of β-cells. Protective effects of MSL-7 against hIAPP oligomer accumulation and hIAPP oligomer-mediated β-cell death are significantly reduced in cells with knockout of MiTF/TFE family members such as Tfeb or Tfe3. MSL-7 improves glucose tolerance and β-cell function of hIAPP+ mice on high-fat diet, accompanied by reduced hIAPP oligomer/amyloid accumulation and β-cell apoptosis. Protective effects of MSL-7 against hIAPP oligomer-mediated β-cell death and the development of diabetes are also significantly reduced by β-cell-specific knockout of Tfeb. These results suggest that an autophagy enhancer could have therapeutic potential against human diabetes characterized by islet amyloid accumulation.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Amyloidogenic peptide oligomer accumulation in autophagy-deficient β cells induces diabetes.J Clin Invest. 2014 Aug;124(8):3311-24. doi: 10.1172/JCI69625. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036705 Free PMC article.
-
Role of Autophagy in the Pathogenesis of Diabetes and Therapeutic Potential of Autophagy Modulators in the Treatment of Diabetes and Metabolic Syndrome.J Korean Med Sci. 2022 Sep 26;37(37):e276. doi: 10.3346/jkms.2022.37.e276. J Korean Med Sci. 2022. PMID: 36163475 Free PMC article. Review.
-
Alpha1-antitrypsin ameliorates islet amyloid-induced glucose intolerance and β-cell dysfunction.Mol Metab. 2020 Jul;37:100984. doi: 10.1016/j.molmet.2020.100984. Epub 2020 Mar 27. Mol Metab. 2020. PMID: 32229246 Free PMC article.
-
UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in β-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy.Autophagy. 2014 Jun;10(6):1004-14. doi: 10.4161/auto.28478. Autophagy. 2014. PMID: 24879150 Free PMC article.
-
Human IAPP amyloidogenic properties and pancreatic β-cell death.Cell Calcium. 2014 Nov;56(5):416-27. doi: 10.1016/j.ceca.2014.08.011. Epub 2014 Aug 27. Cell Calcium. 2014. PMID: 25224501 Review.
Cited by
-
Targeting pancreatic beta cell death in type 2 diabetes by polyphenols.Front Endocrinol (Lausanne). 2022 Nov 17;13:1052317. doi: 10.3389/fendo.2022.1052317. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36465657 Free PMC article. Review.
-
Insulin Resistance and Diabetes Mellitus in Alzheimer's Disease.Cells. 2021 May 18;10(5):1236. doi: 10.3390/cells10051236. Cells. 2021. PMID: 34069890 Free PMC article. Review.
-
Mechanisms of Beta-Cell Apoptosis in Type 2 Diabetes-Prone Situations and Potential Protection by GLP-1-Based Therapies.Int J Mol Sci. 2021 May 18;22(10):5303. doi: 10.3390/ijms22105303. Int J Mol Sci. 2021. PMID: 34069914 Free PMC article. Review.
-
Human islet amyloid polypeptide-induced β-cell cytotoxicity is linked to formation of α-sheet structure.Protein Sci. 2024 Feb;33(2):e4854. doi: 10.1002/pro.4854. Protein Sci. 2024. PMID: 38062941 Free PMC article.
-
TFEB-mediated lysosomal exocytosis alleviates high-fat diet-induced lipotoxicity in the kidney.JCI Insight. 2023 Feb 22;8(4):e162498. doi: 10.1172/jci.insight.162498. JCI Insight. 2023. PMID: 36649084 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

