Fibrillar pharmacology of functionalized nanocellulose
- PMID: 33420138
- PMCID: PMC7794391
- DOI: 10.1038/s41598-020-79592-5
Fibrillar pharmacology of functionalized nanocellulose
Abstract
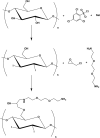
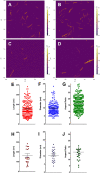
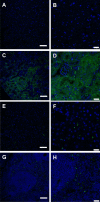
Cellulose nanocrystals (CNC) are linear organic nanomaterials derived from an abundant naturally occurring biopolymer resource. Strategic modification of the primary and secondary hydroxyl groups on the CNC introduces amine and iodine group substitution, respectively. The amine groups (0.285 mmol of amine per gram of functionalized CNC (fCNC)) are further reacted with radiometal loaded-chelates or fluorescent dyes as tracers to evaluate the pharmacokinetic profile of the fCNC in vivo. In this way, these nanoscale macromolecules can be covalently functionalized and yield water-soluble and biocompatible fibrillar nanoplatforms for gene, drug and radionuclide delivery in vivo. Transmission electron microscopy of fCNC reveals a length of 162.4 ± 16.3 nm, diameter of 11.2 ± 1.52 nm and aspect ratio of 16.4 ± 1.94 per particle (mean ± SEM) and is confirmed using atomic force microscopy. Size exclusion chromatography of macromolecular fCNC describes a fibrillar molecular behavior as evidenced by retention times typical of late eluting small molecules and functionalized carbon nanotubes. In vivo, greater than 50% of intravenously injected radiolabeled fCNC is excreted in the urine within 1 h post administration and is consistent with the pharmacological profile observed for other rigid, high aspect ratio macromolecules. Tissue distribution of fCNC shows accumulation in kidneys, liver, and spleen (14.6 ± 6.0; 6.1 ± 2.6; and 7.7 ± 1.4% of the injected activity per gram of tissue, respectively) at 72 h post-administration. Confocal fluorescence microscopy reveals cell-specific accumulation in these target tissue sinks. In summary, our findings suggest that functionalized nanocellulose can be used as a potential drug delivery platform for the kidneys.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

