Neonatal mice resist Plasmodium yoelii infection until exposed to para-aminobenzoic acid containing diet after weaning
- PMID: 33420157
- PMCID: PMC7794322
- DOI: 10.1038/s41598-020-79703-2
Neonatal mice resist Plasmodium yoelii infection until exposed to para-aminobenzoic acid containing diet after weaning
Abstract
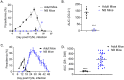
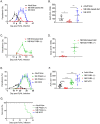
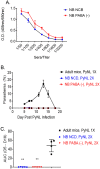
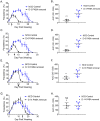
We developed a newborn (NB) mouse Plasmodium yoelii NL infection model to study malaria in early age. Surprisingly, the onset of parasitemia in P. yoelii challenged NB mice was delayed compared to adults and coincided with the weaning date when weanlings switched from maternal milk to normal chow diet. Also, compared to adult mice, parasitemia resolved much later (48 days vs 20 days post challenge) and the peak parasitemia was twice as high in weanlings. Concurrently, weanlings' germinal center reaction was delayed and diminished compared to adult mice. Maternal milk is deficient in para-aminobenzoic acid (PABA), which is required for de novo folate synthesis by Plasmodium. Suggesting a possible role for the protection afforded by PABA-deficient maternal milk, mice fed with a PABA-deficient diet after the weaning continued to control parasitemia. Despite the reduced parasitemia, these mice developed robust T follicular helper (Tfh) responses and were protected from a second P. yoelii challenge. The NB malaria model provides mechanistic insight into the human infant malaria manifestations where a diet solely based on breast-feeding reduces the incidence of severe malaria in infants. NB mice experiments also support further studies to investigate dietary PABA restriction in the management of severe malaria in infants.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- WHO. World Malaria Report 2018. http://www.who.int/malaria/publications/world_malaria_report_2018/report.... (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

