Synergistic antibacterial activity of surfactant free Ag-GO nanocomposites
- PMID: 33420190
- PMCID: PMC7794585
- DOI: 10.1038/s41598-020-80013-w
Synergistic antibacterial activity of surfactant free Ag-GO nanocomposites
Abstract
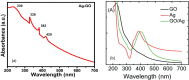
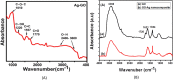
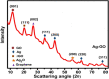
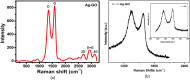
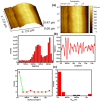
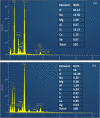
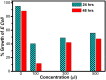
Graphene oxide-silver (Ag-GO) nanocomposite has emerged as a vital antibacterial agent very recently. In this work, we report a facile one step route of Ag-GO nanocomposite formation excluding the aid of surfactants and reductants and was successfully applied to negative Escherichia Coli (E coli) to investigate antibacterial activity by varying doze concentration. The successful formation of Ag-GO nanocomposite via facile one step route was confirmed using Fourier transform infrared spectroscopy (FTIR) and Raman Spectroscopy. The absorption spectra (peak ~ 300 nm) for GO and the (peak ~ 420 nm) for silver nanoparticles were observed. XRD study confirmed the formation of Ag-GO nanocomposite while atomic force microscopy (AFM) showed crumbled GO sheets decorated with Ag nanoparticles. It was observed that the functional groups of GO facilitated the binding of Ag nanoparticles to GO network and enhanced the antibacterial activity of the nanocomposite.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

