Effects of aerobic exercise training on muscle plasticity in a mouse model of cervical spinal cord injury
- PMID: 33420246
- PMCID: PMC7794462
- DOI: 10.1038/s41598-020-80478-9
Effects of aerobic exercise training on muscle plasticity in a mouse model of cervical spinal cord injury
Abstract
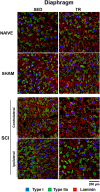
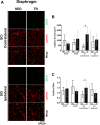
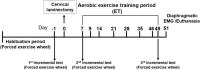
Cervical spinal cord injury (SCI) results in permanent life-altering motor and respiratory deficits. Other than mechanical ventilation for respiratory insufficiency secondary to cervical SCI, effective treatments are lacking and the development of animal models to explore new therapeutic strategies are needed. The aim of this work was to demonstrate the feasibility of using a mouse model of partial cervical spinal hemisection at the second cervical metameric segment (C2) to investigate the impact of 6 weeks training on forced exercise wheel system on locomotor/respiratory plasticity muscles. To measure run capacity locomotor and respiratory functions, incremental exercise tests and diaphragmatic electromyography were done. In addition, muscle fiber type composition and capillary distribution were assessed at 51 days following chronic C2 injury in diaphragm, extensor digitorum communis (EDC), tibialis anterior (TA) and soleus (SOL) muscles. Six-week exercise training increased the running capacity of trained SCI mice. Fiber type composition in EDC, TA and SOL muscles was not modified by our protocol of exercise. The vascularization was increased in all muscle limbs in SCI trained group. No increase in diaphragmatic electromyography amplitude of the diaphragm muscle on the side of SCI was observed, while the contraction duration was significantly decreased in sedentary group compared to trained group. Cross-sectional area of type IIa myofiber in the contralateral diaphragm side of SCI was smaller in trained group. Fiber type distribution between contralateral and ipsilateral diaphragm in SCI sedentary group was affected, while no difference was observed in trained group. In addition, the vascularization of the diaphragm side contralateral to SCI was increased in trained group. All these results suggest an increase in fatigue resistance and a contribution to the running capacity in SCI trained group. Our exercise protocol could be a promising non-invasive strategy to sustain locomotor and respiratory muscle plasticity following SCI.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Permanent diaphragmatic deficits and spontaneous respiratory plasticity in a mouse model of incomplete cervical spinal cord injury.Respir Physiol Neurobiol. 2021 Feb;284:103568. doi: 10.1016/j.resp.2020.103568. Epub 2020 Nov 2. Respir Physiol Neurobiol. 2021. PMID: 33144274
-
Protein Tyrosine Phosphatase σ Inhibitory Peptide Promotes Recovery of Diaphragm Function and Sprouting of Bulbospinal Respiratory Axons after Cervical Spinal Cord Injury.J Neurotrauma. 2020 Feb 1;37(3):572-579. doi: 10.1089/neu.2019.6586. Epub 2019 Sep 18. J Neurotrauma. 2020. PMID: 31392919 Free PMC article.
-
Intraspinal microstimulation and diaphragm activation after cervical spinal cord injury.J Neurophysiol. 2017 Feb 1;117(2):767-776. doi: 10.1152/jn.00721.2016. Epub 2016 Nov 23. J Neurophysiol. 2017. PMID: 27881723 Free PMC article.
-
Prolonged acute intermittent hypoxia improves forelimb reach-to-grasp function in a rat model of chronic cervical spinal cord injury.Exp Neurol. 2021 Jun;340:113672. doi: 10.1016/j.expneurol.2021.113672. Epub 2021 Feb 27. Exp Neurol. 2021. PMID: 33652030 Review.
-
The challenges of respiratory motor system recovery following cervical spinal cord injury.Prog Brain Res. 2014;212:173-220. doi: 10.1016/B978-0-444-63488-7.00010-0. Prog Brain Res. 2014. PMID: 25194199 Review.
Cited by
-
Effects of C2 hemisection on respiratory and cardiovascular functions in rats.Neural Regen Res. 2023 Feb;18(2):428-433. doi: 10.4103/1673-5374.346469. Neural Regen Res. 2023. PMID: 35900441 Free PMC article.
-
Therapeutic Strategies Targeting Respiratory Recovery after Spinal Cord Injury: From Preclinical Development to Clinical Translation.Cells. 2023 May 31;12(11):1519. doi: 10.3390/cells12111519. Cells. 2023. PMID: 37296640 Free PMC article. Review.
-
Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats.Biology (Basel). 2022 Mar 19;11(3):473. doi: 10.3390/biology11030473. Biology (Basel). 2022. PMID: 35336846 Free PMC article.
-
Contrasting Experimental Rodent Aftercare With Human Clinical Treatment for Cervical Spinal Cord Injury: Bridging the Translational "Valley of Death".J Neurotrauma. 2023 Dec;40(23-24):2469-2486. doi: 10.1089/neu.2023.0314. Epub 2023 Nov 10. J Neurotrauma. 2023. PMID: 37772694 Free PMC article. Review.
-
When Spinal Neuromodulation Meets Sensorimotor Rehabilitation: Lessons Learned From Animal Models to Regain Manual Dexterity After a Spinal Cord Injury.Front Rehabil Sci. 2021 Dec 7;2:755963. doi: 10.3389/fresc.2021.755963. eCollection 2021. Front Rehabil Sci. 2021. PMID: 36188826 Free PMC article. Review.
References
-
- Komnenov D, Solarewicz JZ, Afzal F, Nantwi KD, Kuhn DM, Mateika JH. Intermittent hypoxia promotes recovery of respiratory motor function in spinal cord-injured mice depleted of serotonin in the central nervous system. J. Appl. Physiol. 2016;121(2):545–557. doi: 10.1152/japplphysiol.00448.2016. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

